The availability of substituted cathinones has grown during the past two decades on the black market for drugs. Typically, these drugs first became well-liked as safe alternatives to amphetamine-like stimulants that are already on the market. As they become more well-known, national governments eventually forbid them, and when they are outlawed, they may be found alongside other regulated substances on the black market for drugs. Mephedrone is one of the more well-known substituted cathinones, and consumption of it has remained prevalent long after it was declared illegal. 4-MMC has quite a few pharmacological similarities to methamphetamine, but the long-term neurotoxic effects of these substances are quite different. Despite the fact that METH is known to cause significant and persistent drops in monoamine levels and other indicators of DA neurotoxicity, similar results are typically not duplicated with 4-MMC. In reality, when delivered under typical circumstances, mephedrone shows no sign of neurotoxicity, according to multiple studies. However, when the medication is delivered under conditions that are known to increase stimulant neurotoxicity, such as high ambient temperatures, 4-MMC neurotoxicity can be accelerated.
It is known that in recreational settings, psychostimulants are often consumed in conjunction with alcohol. Alcohol use is a known exacerbating factor for stimulant neurotoxicity and has been shown to increase or precipitate neurotoxic effects of MDMA and METH. The possibility that alcohol may possibly cause 4-MMC neurotoxicity is therefore raised. Currently, not much is understood about this. When 4-MMC and EtOH were delivered together in one trial, there was evidence of neurotoxicity; however, because the medication was also provided at high ambient temperatures in this study, it is difficult to definitively assign the effects to EtOH. It’s interesting to note that a clinical investigation on the acute effects of 4-MMC alone or in combination with EtOH found that EtOH boosted the drug’s cardiovascular effects and self-reported pleasure. This study did not, however, look into the long term. Given that 4-MMC and EtOH are frequently combined in drug use, more research into this issue is crucial. It will assist both drug users and healthcare professionals understand the hazards involved and direct the creation of an evidence-based harm reduction strategy.
In a previous study, we used manganese-enhanced magnetic resonance imaging to compare the long-term effects of 4-MMC and METH in rats. We found that 2-weeks after a binge-dosing regimen, METH produced a pattern of widespread neuronal deactivation in monoamine-rich brain areas; in contrast, 4-MMC produced an effect that was restricted primarily to the parietal cortex, hypothalamus, and hippocampus. In a prior work, MPTP-induced loss of DA neurons in the substantia nigra resulted in reduced neuronal activation by MEMRI, which was related with neurotoxicity. The MEMRI method is based on the observation that manganese performs the same function as Ca2+ analogue in vivo; manganese influx into neuronal cells via Ca2+ channels during rapid neuronal depolarization and subsequent distribution throughout the neuron and axons thus represent a measure of mean neuronal activity over time.
Materials and methods used in this study
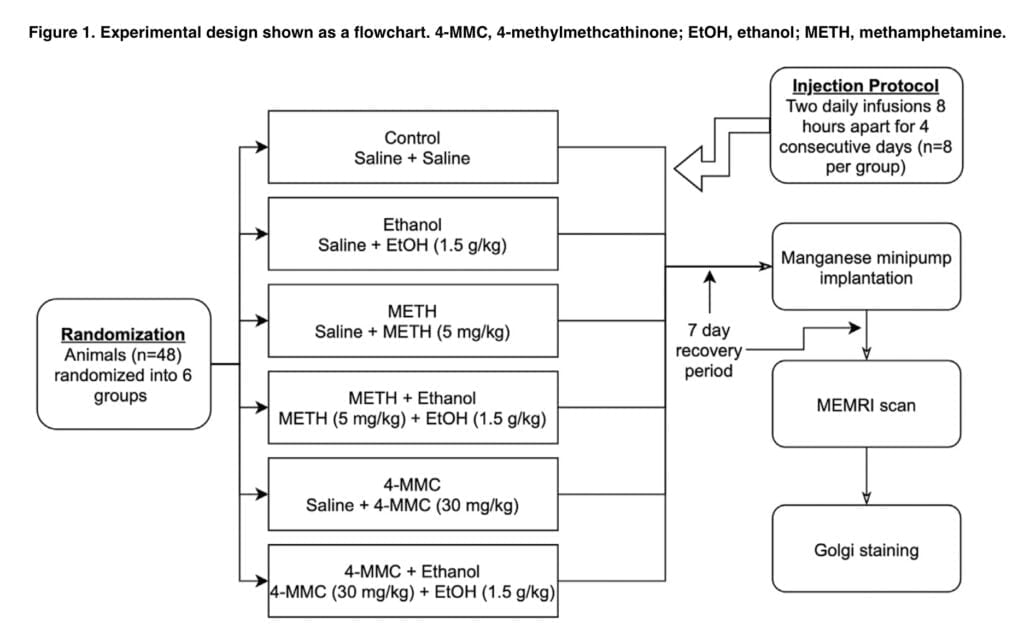
Our experiment was conducted using 48 young male rats that were randomly divided into six groups of 8 specimens each. The substances used were dissolved in saline and injected intraperitoneally at a dose of 1 ml/kg (for mephedrone or methamphetamine) and 10 ml/kg (for ethanol) at 8-hour intervals. The dosages of 5 mg/kg METH and 30 mg/kg 4-MMC utilized in this investigation were comparable to those previously used and were calculated based on an estimate of the relative potency difference between 4-MMC and METH. The blood alcohol level of 0.2% that is typical of dangerous binge drinking was achieved with the 1.5 g/kg EtOH dosage. An overview of drug and EtOH treatments administered to the various experimental groups is shown in Figure 1. The first day of the experiment was started with weight estimation of the animal, and then weight estimation was performed after the last drug intake on the fourth day of the experiment. Thermometry was performed 30 minutes after the first intake of the substance. Seven days after the last drug administration, animals were injected with manganese dichloride by implantation of osmotic minipumps. On the 18th day of the experiment, the animals were subjected to MEMRI brain imaging. MEMRI imaging was performed in a horizontal magnet using a volume coil transmitter/4-channel surface coil receiver pair. During the experiments, the animals were anesthetized with isoflurane and placed inside a holder with breath monitoring and temperature control using warm water. T1-weighted images were acquired using a three-dimensional rapid acquisition-relaxation enhanced pulse sequence.
T1-weighted brain images were standardized according to the template of the rat brain, co-registered with figures from the atlas. Voxel-wise independent two-tailed t-tests were used to compare the groups in order to create statistical parametric maps of the differences in brain activity between the experimental groups. Systemic delivery can result in inter-individual variances in Mn2+ accumulation in the brain, resulting changes in mean global signal intensity across individuals despite the MnCl2 concentration for each individual rat being adjusted depending on body weight. Therefore, the mean global intensity was included as a covariate in the general linear model on a voxel-by-voxel. To describe further activation differences between the groups at anatomically specified brain areas, three- dimensional masks were. For comparing the groups across selected ROIs, mean ROI intensity values were extracted including all voxels within the ROIs in the analysis.
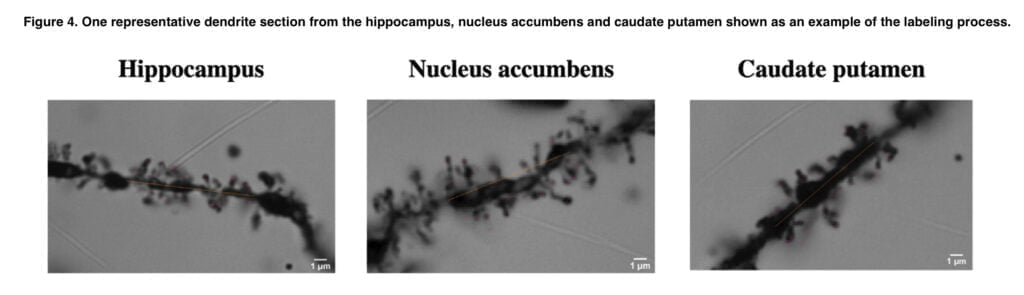
Animals were sacrificed by decapitation once the MRI scan was finished, and the brains were then extracted. The brain tissues were then stained using a commercial Golgi staining kit. The extracted brains were essentially submerged in an impregnation solution. The brains were impregnated for 14 days before being frozen in isopentane and kept at a temperature of 80°C. The brains were then sectioned into 100-μm-thick coronal sections and stained in a multistep staining protocol on gelatin-coated slides. The correct brain areas were determined throughout the imaging procedure, and z-stacks were then collected from each region, generally from the same point inside the area, maintaining the order of the dendritic branch (second to third). The imaging was performed blindly, so that the researcher did not know the treatment group of individual sections.
Discussion of the results
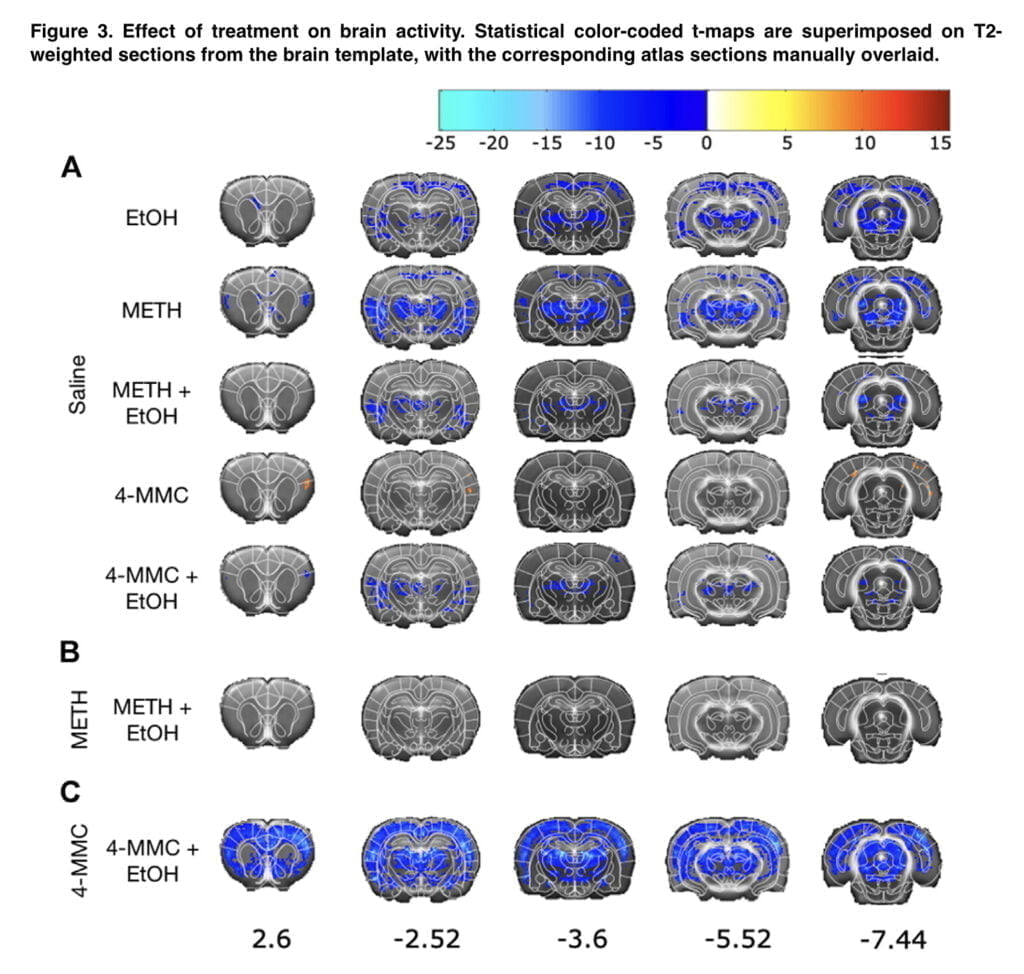
Here, we demonstrate that, when examined with MEMRI two weeks after the last dose of the drugs, co-administration of EtOH with 4-MMC in a binge-abuse paradigm results in a broad pattern of neuronal deactivation in monoamine-rich brain locations. The pattern of 4-MMC + EtOH deactivation was strikingly similar to that seen after METH treatment. Contrary to the outcome observed when 4-MMC was given alone, this pattern of broad deactivation. 4-MMC increased, rather than decreased, neuronal activity in an anatomically more limited manner, and occurring in areas not strongly innervated by DA axons. These findings regarding 4-MMC alone were in line with our previous results, indicating good reproducibility of the MEMRI method. We did not discover any morphological alterations to the dendrites that were suggestive of long-term neurotoxic effects when we looked at the neuronal morphology in the hippocampus, nucleus accumbens, and caudate-putamen, the three areas showing the strongest neuronal deactivation in animals treated with 4-MMC and EtOH.
When the animal was injected with mephedrone only, we observed a pattern of long-term neuronal activation in the following areas: retrosplenial and primary somatosensory cortex, dorsal hippocampus, thalamic areas, and superior colliculus. These areas are not strongly innervated by DA, and the observed changes plausibly do not reflect DA neurotoxicity. Plasma cortisol levels have been reported to increase during intoxication with 4-MMC, which may be a possible reason for the enhanced neuronal activity. It has been demonstrated that areas necessary for memory consolidation, such as the hippocampus, are activated when cortisol levels are elevated in response to acute stress via the serotonergic effects on the hypothalamo-pituitary-adrenal axis. We found it interesting that 4-MMC did not cause a decrease in monoamine levels (which would indicate toxicity), but did decrease memory scores 2 weeks after ingestion.
Given the anatomical correlation between the deactivation pattern and brain regions innervated by DA nerve endings and frequently associated with amphetamine neurotoxicity, the pattern of extensive neuronal deactivation observed following treatment with 4-MMC combined with EtOH as well as following METH, either alone or in combination with EtOH, is startling. However, further Golgi staining and dendritic spine analyses found no evidence of neurotoxicity, even in the regions with the highest deactivation. In high ambient temperatures, co-administration of 4-MMC with EtOH increases oxidative stress markers while decreasing synaptic transmitter function markers for DA and 5-HT. It is thus plausible that the enhanced oxidative stress-related adaptation in synaptic terminals has long-term functional repercussions that are reflected in the deactivation caused by 4-MMC and EtOH that was found here. Considering that the EtOH dose employed in this study was moderate it is worth noting that neuronal deactivation as assessed by MEMRI should not be considered as conclusive evidence of neurotoxicity, although such a correlation has been observed previously. Similar to the 4-MMC group, neurotoxicity could have happened in a way that isn’t apparent from the MEMRI data. Until the precise neurochemical correlates of changed activation patterns are known, the results must be taken with some degree of care.
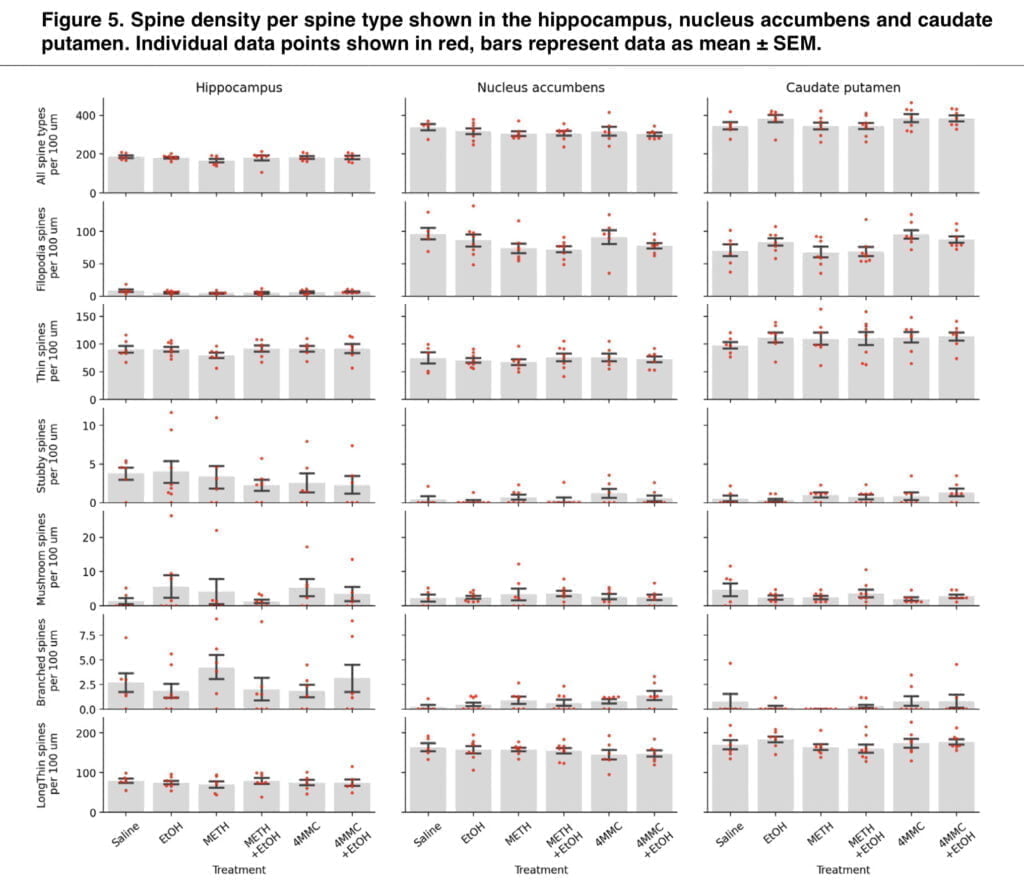
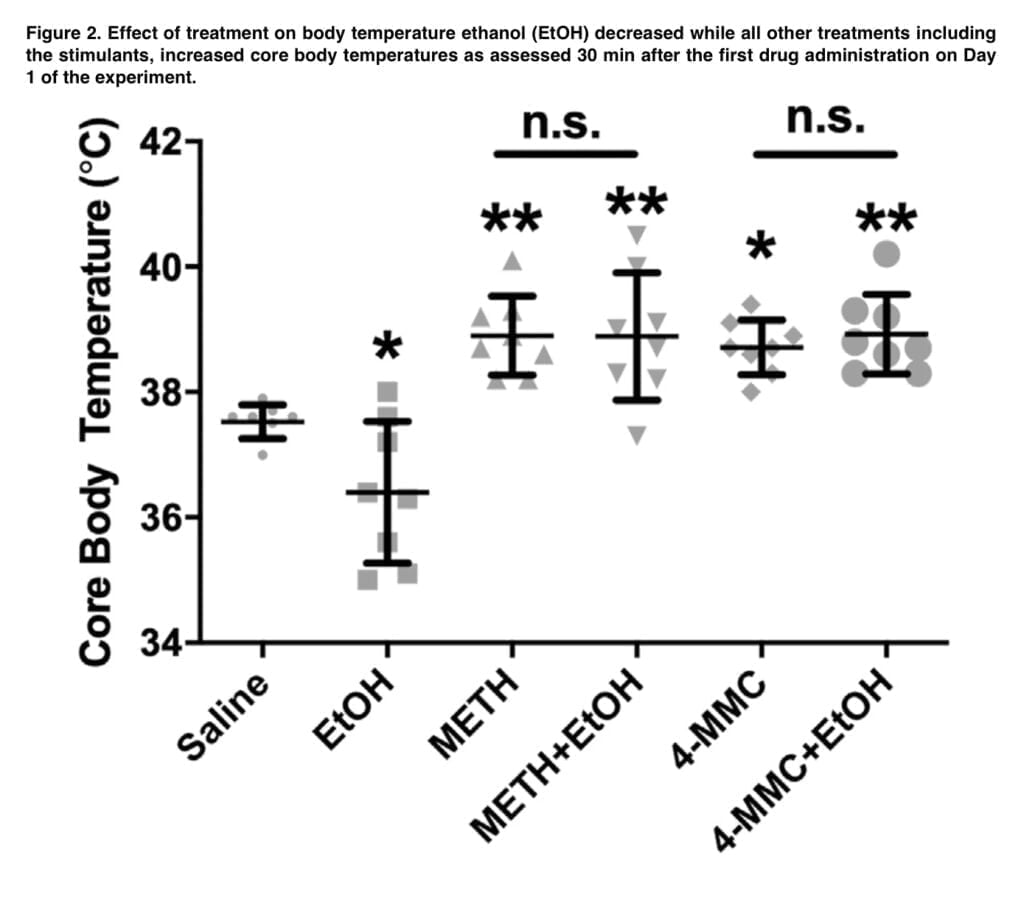
In keeping with findings from other research, this study found that core body temperatures were lower in the EtOH group than in the control group, but higher in the 4-MMC and METH groups. Notably, the hypothermic effects of 4-MMC and METH were not increased nor inhibited by the co-administration of EtOH. Any variations between groups in this study were not caused by a different hyperthermic reaction because hyperthermia was present in groups treated with stimulants and was unaffected by EtOH co-administration. Golgi staining followed by spine analysis has been suggested to be an effective and unbiased way to assess neuronal changes at the dendritic spine level. However, in the present experiment, no statistically significant alterations were found in the spine analysis in three relevant brain regions. This lack of modifications may be attributed to the limits of Golgi staining, including variability in staining intensity, inconsistency in localizing the brain region within thick brain slices, or the intrinsic randomness of the Golgi method’s labeling of distinct kinds of neurons. To our knowledge there has been no previous research to assess the temporal stability of stimulant-induced alterations in spine morphology. It is possible that stimulant-induced alterations in spine morphology are not stable enough to persist following the 2-weeks recovery period after treatments. Furthermore, Golgi-staining only affects the post-synaptic portions of the synapses, whereas stimulant-induced neurotoxicity has previously been linked to the axonal portion of the synapses, but at larger dosages than those employed in this work, starting at 20 mg/kg and higher. And as a result, it’s possible that our dosage of 5mg/kg METH wasn’t enough to trigger axonal death, which would subsequently result in dendritic spine distortion.
Conclusion
When 4-MMC is delivered alone, the effects on neuronal activity are minimal, physically restricted, and probably not indicative of long-term neurochemical changes. However, when combined with EtOH, it results in a pattern of extensive neuronal deactivation in areas of the brain that are heavily innervated by DA neurons, similar to what happens after METH. Despite this, there are no obvious morphological alterations in the deactivated areas that would indicate toxicity. This indicates that the deactivation pattern is probably caused by long-term neurochemical changes that are significant but not severe enough to manifest as overt neurotoxicity. In conclusion, this study suggests that binge-treatment with 4- MMC on its own does not substantially alter brain activity, but in combination with EtOH may result in long-term reductions in brain activity. This is important from a harm-reduction perspective, although further studies are needed to outline the exact neurochemical changes responsible for the altered activity patterns.
