The role of dopamine in the neurotoxicity of mephedrone
Mephedrone (4-methylmethcathinone) is a synthetic cathinone derivative that is structurally and pharmacologically related to the psychostimulants 3,4-methylenedioxymethamphetamine and has been classified as a new psychoactive substance. Mephedrone is abused by club drug users, predominantly adolescents and young adults, in a binge-like fashion (“stacking”). Mephedrone increases dopamine levels in the body: this means that the neurotoxicity of mephedrone is directly related to the amount of dopamine, which is highly variable with single MCAT use.
Published data indicate that most mephedrone users are engaged in “heavy” alcohol use immediately prior to consuming mephedrone, and then they reduce alcohol consumption as the effects of mephedrone are experienced during the drug episode. The drug cocktail bath salts typically contains the mephedrone, despite being a prohibited chemical with a high abuse potential. However, acute toxicity and sporadic fatalities have also been observed.

Mephedrone increases dopamine levels in the body
Users of the substance describe subjective effects including feelings of pleasure, well-being, and changed sensory experiences. Comparatively to methamphetamine (METH), its non-b-keto analog, mephedrone has a noticeably comparable neurotransmitter releasing impact on dopamine (DA) and serotonin (5-HT), mediated by their respective reuptake transporters. Additionally, mephedrone shares similarities with METH in its acute effects on thermoregulation, locomotor stimulation. Despite these similarities, these two compounds differ in their ability to evoke long-term toxicity to DA nerve endings.
The traditional amphetamines, including METH, have a well-established history of causing DA nerve terminal injury in rat studies. Reduced levels of dopamine transporter (DAT), tyrosine hydroxylase, and other indicators of presynaptic dopaminergic integrity are frequently seen with METH (TH).
This toxicity is assumed to be mediated by neuroinflammatory and oxidative stress-related mechanisms. Due to the fact that DA’s metabolism and auto-oxidation are known to produce reactive species that may contribute to neuronal damage, it has been suggested that the excessive DA release induced by METH is a key culprit. In general, it has not been seen that mephedrone causes this persistent dopaminergic toxicity.
Except in more extreme environmental circumstances, the majority of rodent investigations under settings known to elicit neurotoxicity with METH have not observed equivalent neurochemical or inflammatory alterations in MEPH-treated animals.
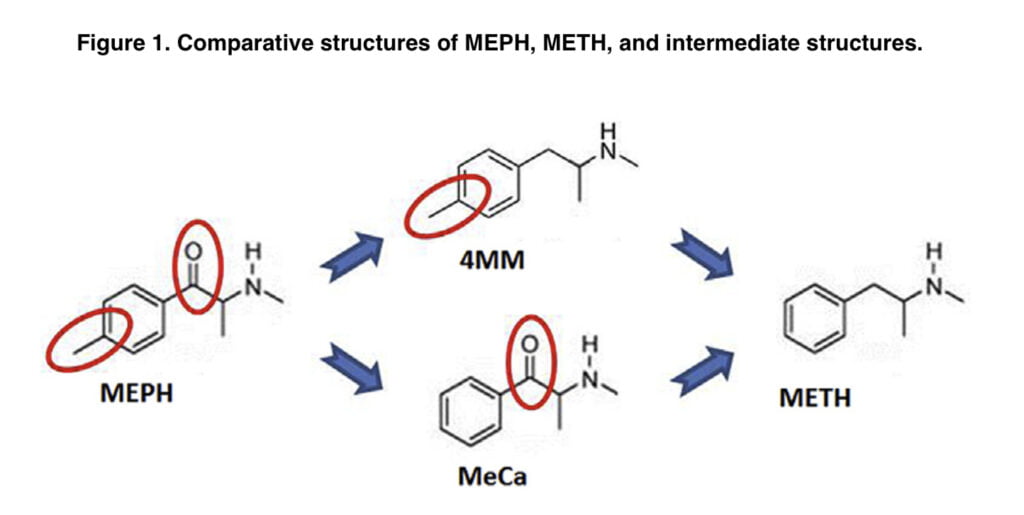
Mephedrone differs from METH by having a 4-methyl group on the phenyl ring and a b-keto group. In this laboratory, a recent experiment examined the toxicity of two intermediate substances, methcathinone and 4-methylmethamphetamine (Figure 1). MeCa, while being less effective, caused dopaminergic toxicity similar to that of METH, whereas 4-MMC resembled MEPH since it caused significantly less dopaminergic toxicity than METH.
It is yet unclear what causes this differential toxicity. Increases in the releasable pool of DA have been proven in several investigations to increase the toxicity of METH. Mephedrone, which is non-toxic, enhances METH toxicity as well and may do so via interactions with the releasable pool of DA, as it has been reported that METH and MEPH both release DA via reverse transport through the DAT in vitro. Also, another goal of our study was to find out how mephedrone increases the concentration of dopamine in the brain.
The inability of MEPH alone to increase the cytosolic, drug-releasable pool of DA in a VMAT2-dependent manner could explain its low neurotoxic potential by comparison to METH, which releases DA from vesicles into the cytosol, and then through the DAT into the synapse. When compared to non-toxic mephedrone, MeCa, which is neurotoxic, released more DA through the DAT. MeCa was observed to release mephedrone (42% vs 33%) in the same investigation, despite having a lower binding affinity and release profile at VMAT2 than METH.
We hypothesized that increasing the drug-releasable pool of dopamine by administering either the dopamine precursor L-DOPA, the monoamine-oxidase (MAO) B inhibitor pargyline, or a mild dose of METH, which can release vesicular dopamine to the cytosol, would impart toxicity to MEPH as well as increase the dopaminergic toxicity of the two closely related structural analogs.
Materials and methods
In this study, we used the following preparations: (R,S)-N-Methcathinone HCl; (R,S)-mephedrone HCl; racemic 4-methlymethamphetamine HCl; (þ)- Methamphetamine HCl; pargyline HCl; L-3,4-dihydroxyphenylalanine (L-DOPA); S-(-)-carbidopa; polyclonal antibodies against glyceraldehyde 3-phosphate dehydrogenase (GAPDH); bicinchoninic acid protein assay kits for Western blot analysis and polyclonal antibodies against rat TH. The method of drug administration to the mice was intravenous. The control group received saline, MeCa was administered at a dose of 80 mg/kg, and mephedrone was administered at a dose of 40 mg/kg at 2-hour intervals.
4-MM, MeCa, and MEPH were administered in conjunction with either METH, which releases vesicular DA into the cytosol, or with L-DOPA or pargyline, which increase the amount of cytosolic DA through increased synthesis or inhibition of MAO-B metabolism, respectively, in order to increase the pool of releasable DA. METH (2.5 mg/ kg) was given simultaneously with saline, 4-MM, MeCa, or MEPH, as this regimen is known to show enhanced toxicity when combined with b-ketoamphetamines.
Pargyline (25 mg/kg) was administered i.p. as a single injection 1 h prior to the first injection of saline, 4-MM, MeCa, or MEPH. 50 mg/kg L-DOPA (50 mg/ kg) and carbidopa was administered 1 h prior to each injection of saline, 4-MMC, MeCa, or MEPH in 0.4 mL of warm distilled water. Mice were put to death 48 hours after the last dose of medication since that is when METH-related elevations in neuroinflammation and reductions in dopaminergic toxicity indicators are known to be at their peak. For all pharmacological treatment tests, the ambient temperature was kept between 22 and 24 C.
The effects of drug treatments on striatal DAT and TH levels, highly specific markers for striatal DA nerve endings, were deter- mined by immunoblotting as an index of toxicity. Striatal tissue was dissected from the brain after treatment and stored at 80 C. Immunoreactive bands were visualized by enhanced fluorescence and the relative densities of TH-, DAT-, and GAPDH-reactive bands were determined by imaging and quantified. DAT and TH relative densities were normalized to the GAPDH level for each lane to control for loading error.
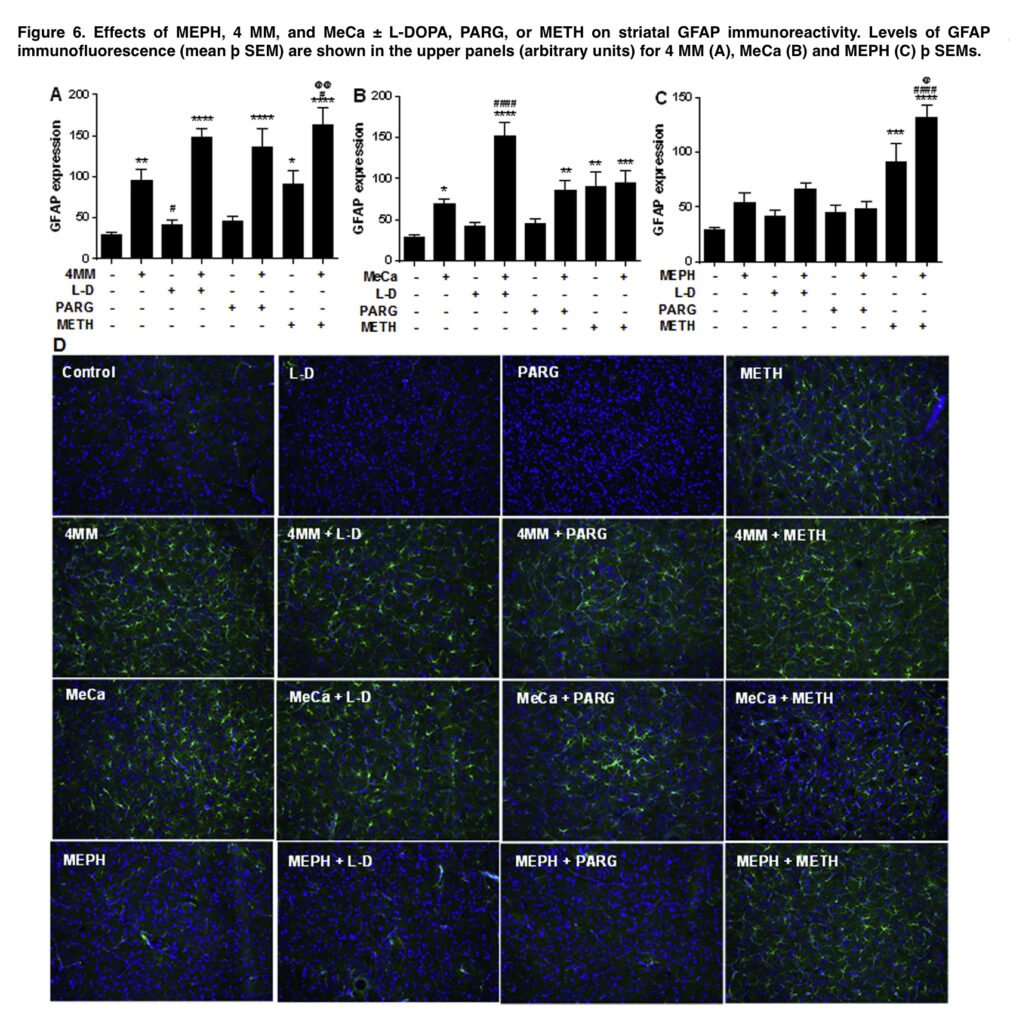
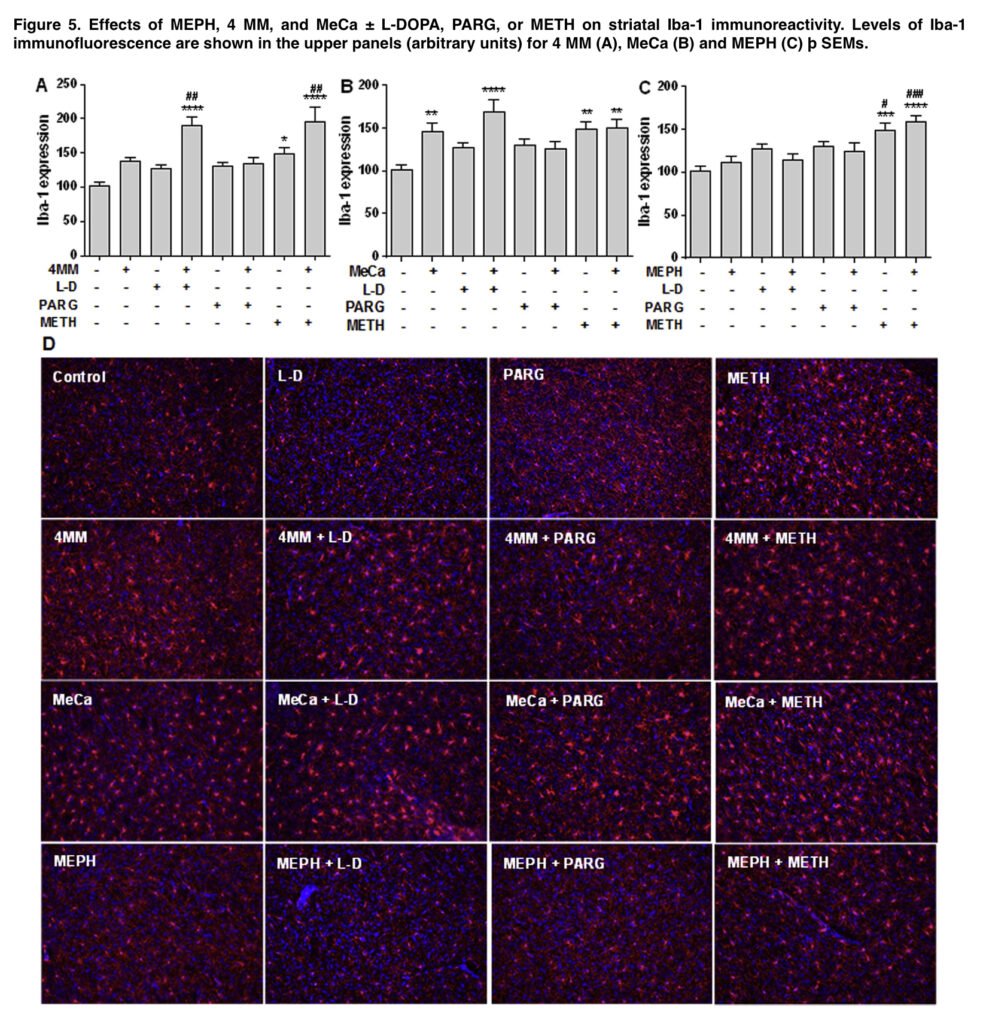
Microglial activation was assessed as before using antiionized calcium-binding adapter molecule 1, and astrocyte activation was assessed as described using antieglial fibrillary acidic protein antibody. Images acquired at 20 magnification from 3 non-adjacent regions of interest per slice from 3 to 5 mice were used for analysis. The results are expressed as mean of fluorescence intensity (arbitrary units). In order to reduce the number of mice used, some groups were utilized in all analyses as these animals were run concurrently with every other treatment.
Discussion of the results
The goal of this study was to test the theory that the variations in dopaminergic neurotoxicity seen in these closely linked structures are caused by variations in the amount of dopamine released after binge treatment with the amphetamine-related substances METH, MEPH, 4-MMС, and MeCa. It was specifically hypothesized that mephedrone lacks dopaminergic toxicity because, in contrast to the releasing effects of the neurotoxic compounds METH and to a lesser extent MeCa, it cannot increase the cytosolic pool of releasable DA from vesicular stores in a VMAT2-dependent manner and has less DA release via the DAT.
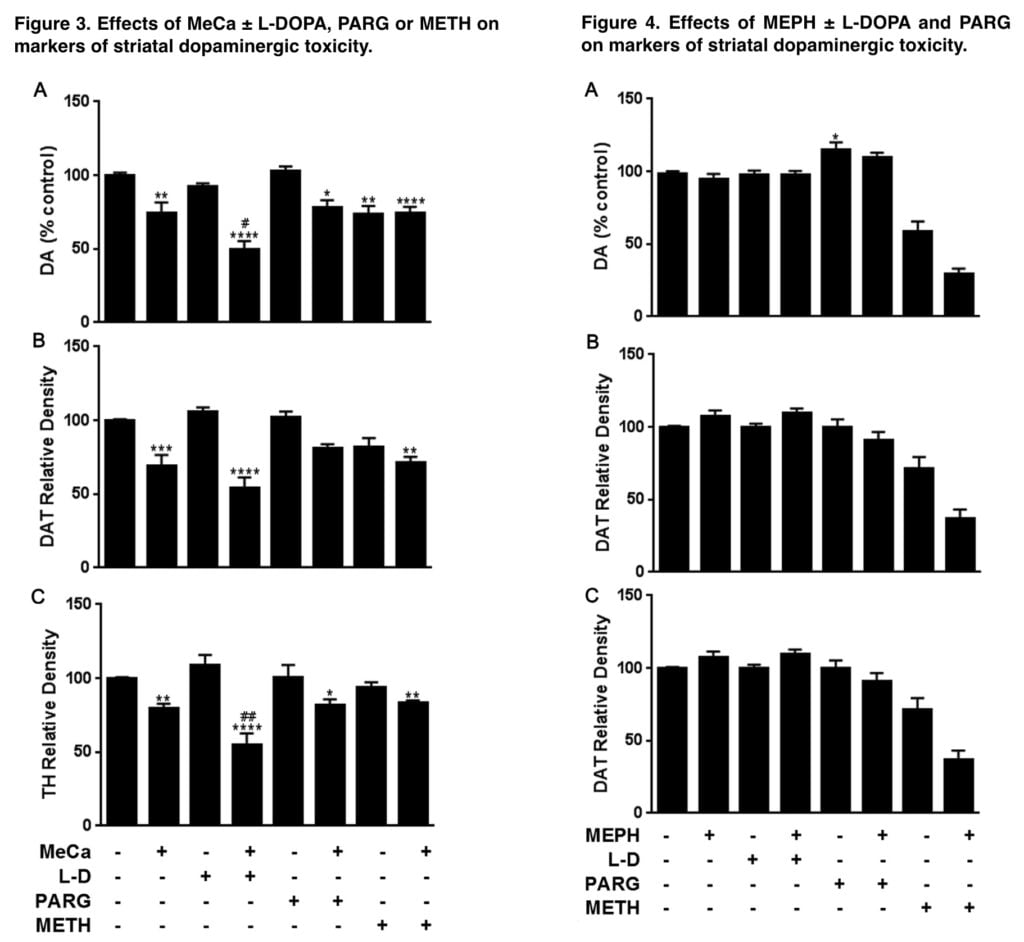
A mildly toxic dose of METH, a well-known vesicular DA releaser into the cytosol, was combined with mephedrone, the structurally related substances 4-MMС and MeCa, as well as 3 treatments known to increase the pool of releasable DA: the DA synthesis precursor L-DOPA, the MAO-B DA metabolism inhibitor pargyline, and the mephedrone. We predicted that the higher levels of releasable DA brought on by these therapies would be more toxic to MEPH, 4-MMС, and MeCa as measured by decreased levels of dopaminergic markers and elevated levels of glial activation.
In line with our hypothesis, all 3 treatments that raised the pool of releasable DA significantly increased the dopaminergic toxicity of 4-MMС. Similarly, the toxicity induced by MeCa was increased when combined with L-DOPA, but neither pargyline nor METH had any effect. There have been earlier reports of significantly increased dopaminergic depletions when MEPH and METH are combined.
When coupled with either L-DOPA or pargyline, mephedrone did not cause any dopaminergic toxicity, in contrast to the intermediate molecules. A tried-and-true approach for raising the DA drug-releasable pool is pretreatment with pargyline or L-DOPA. Numerous studies have demonstrated that the DA precursor L-DOPA significantly raises striatal DA concentrations by 50–70%.
When combined with amphetamine, it has also been demonstrated to greatly increase striatal DA release. These results show that striatal DA concentrations and amphetamine-stimulated DA efflux are both increased by the DA-enhancing therapies used in the current investigation.
Given the closeness of L-toxic DOPA’s profile to that of 4-MMC and the observed responsiveness of 4-MM to the dopamine-enhancing drugs in this work, the failure of L-DOPA or pargyline to impart dopaminergic toxicity to MEPH was unexpected. Only the highest dosage in the range studied for 4-MMC in our previous study resulted in substantial DA and DAT depletions, closely resembling the absence of toxicity found in MEPH.
Given that the total level of MeCa toxicity was closer to METH, it was concluded that the ring substitution in MEPH had a stronger reductive influence on toxicity than the b-keto substitution. L-DOPA and pargyline, however, increase the 4-MMC toxicity, which is a trait shared by METH but not MEPH. As a result, it appears that the connection between certain substituents and dopaminergic neurotoxicity in these chemicals is more nuanced than first thought.
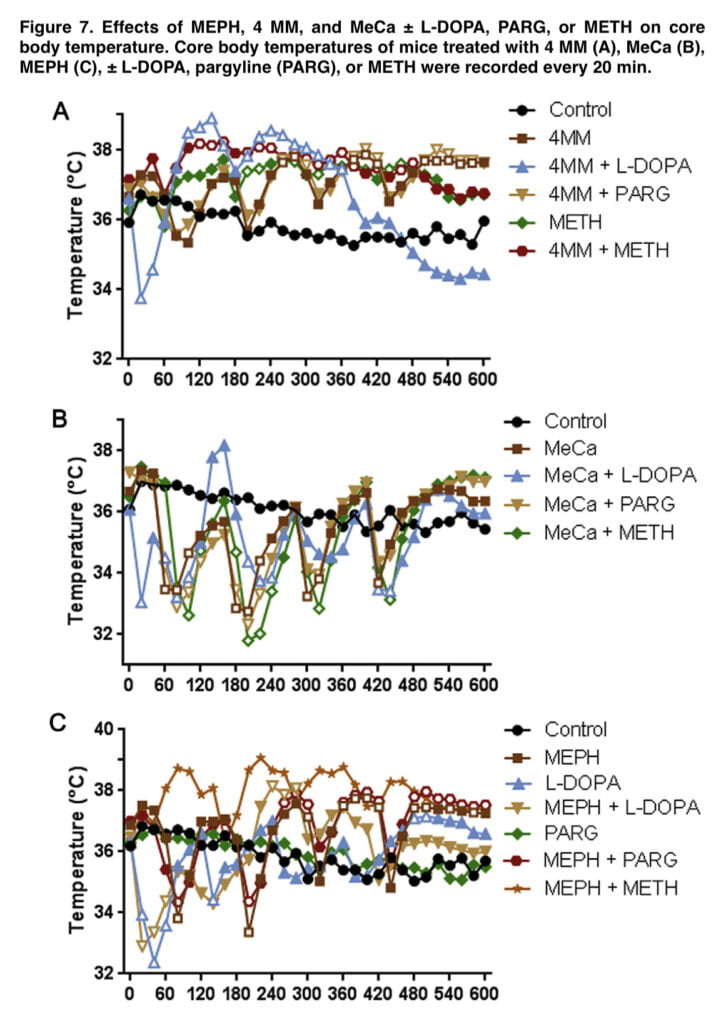
The final experiment of this investigation investigated the effects of an enlarged pool of releasable DA on the body temperature effects of 4-MMC, MeCa, and MEPH to determine any possible influence on toxicity. It has been well known that increases in associated hyperthermia can significantly change METH toxicity. These substances, when administered alone, had dramatically different impacts on core body temperatures, which was consistent with other observations.
While MeCa caused severe, protracted hypothermia with each injection, 4-MMC caused heat. After the first two injections, MEPH-treated rats displayed a brief hypothermia, which was followed by a persistent heat (Figure 7). It’s interesting to note that L-DOPA alone, as well as when combined with 4-MMC, MeCa, and MEPH, produced an acute, temporary hypothermia that lasted for 20 to 40 minutes after the initial injection.
Although the duration of either hyperthermia in 4-MMC and MEPH or hypothermia in MeCa was reduced when L-DOPA was combined with them, these effects were still statistically significant when compared to controls. Although amphetamine toxicity can be abated by inducing hypothermia, it is doubtful that L-brief DOPA’s hypothermia interfered with any potential MEPH toxicity in our investigation.
Conclusion
Although the exact mechanism underlying these effects is still unknown, it is obvious that the structural substituents in these molecules significantly affect how each medicine affects body temperature. It is improbable that the dopaminergic system alone causes these observed differences given the typically limited influence of increased DA on the drug-induced temperature changes in our investigation.
One group used 5-HT depletion and 5HT1A receptor antagonists to counteract the first hypothermic effects of mephedrone, while a recent study discovered evidence for adrenergic regulation of mephedrone hyperthermia in rats. Further research on the body temperature effects of MeCa and 4-MMC is required to demonstrate that the observed differences in body temperature effects across bath salts involve various neurotransmitter pathways.
Despite long being believed to be necessary for METH toxicity, hyperthermia was not clearly correlated with dopaminergic depletions in the current investigation, which is significant. MeCa depleted DA, DAT, and TH in the striatum and caused substantial increases in glial reactivity both when administered alone and when combined with improved releasable DA, but typically hyperthermic mephedrone showed little toxicity and did not raise glial reactivity. This separation supports the idea that hyperthermia is only a contributing component, not a causative one, in METH poisoning.
Despite a corpus of work devoted to explaining why mephedrone does not cause dopaminergic neurotoxicity in the same way that METH does, the underlying mechanism(s) involved are still unknown. Finding this mechanism will help us better understand the neurotoxicity caused by METH and might help us develop a cure for the long-term effects of METH exposure.
