To date, there have been virtually no studies evaluating the acute effects and pharmacokinetics of mephedrone in humans through oral or intranasal use. There is now some evidence based on preclinical models using mephedrone that shows that mephedrone causes an increase in psychomotor and locomotor activity, which are typical properties of psychostimulants. However, apart from sporadic publications with episodes of self-administration, as well as informal reports in forums, there has been no research to date using both subjective and objective criteria. Various studies on the metabolic activity of mephedrone (including the use of human body fluids) demonstrate that, in most cases, mephedrone is metabolized by the CYP2D6 isoenzyme. Despite the increasing use of mephedrone, scientific knowledge of the acute effects and the pharmacodynamics and pharmacokinetics of 4-mmc is severely limited.
This publication describes an observational study with ten healthy, experienced drug users (4 females and 6 males) who self-administered a single oral dose of mephedrone. In general, information on the effects of mephedrone on humans is extremely limited in the scientific world. Various parameters of cardiovascular system and homeostasis were studied, evaluation criteria from a set of visual analogue scales, ARCI and VESSPA-SEE questionnaires were used. The main purpose of the present study is to compare the acute effects of mephedrone after oral and intranasal use under observational conditions close to reality.
General results of the evaluation scales
In terms of physiological effects, both oral and intranasal mephedrone use result in an increase in SBP, DBP, HR and T (see attached Table 1). A comparative analysis of the two routes of administration showed no significant differences for E, AUC and T-C, but there is a difference in SBP, DBP and HR 1 and 2 hours after administration depending on the route of administration. Regarding subjective effects, a comparison of the two routes of administration revealed significant differences for indicators such as Emax and AUC0-4 h.
[table id=1 /]
Emax = peak effects 0–4 h (differences from baseline); AUC0–4 = Area under the curve 0–4 h; Emax measured by C (T (temperature)) mm (visual analog scale (VAS)), and score (Addiction Research Center Inventory (ARCI), Evaluation of Subjective Effects of Substances with Abuse Potential questionnaire (VESSPA-SEE)), and expressed as mean and standard deviation. A p-value < 0.05 was considered statistically significant.
After oral mephedrone, T-C comparison to baseline showed significant differences for intensity, stimulates, high and good effects at 1 and 2 h, whilst for liking and content, differences were detected in all times evaluated. In contrast, after intranasal self-administration T-C comparison to baseline only showed significant differences for intensity, stimulates, high, good effects, and content at 1 h and for liking at 1 and 2 h, respectively. Both routes of 4-mmc administration caused mild perceptual changes but no hallucinations, although no statistically significant differences were found between the routes of administration, except for body feeling (AUC0-4h).
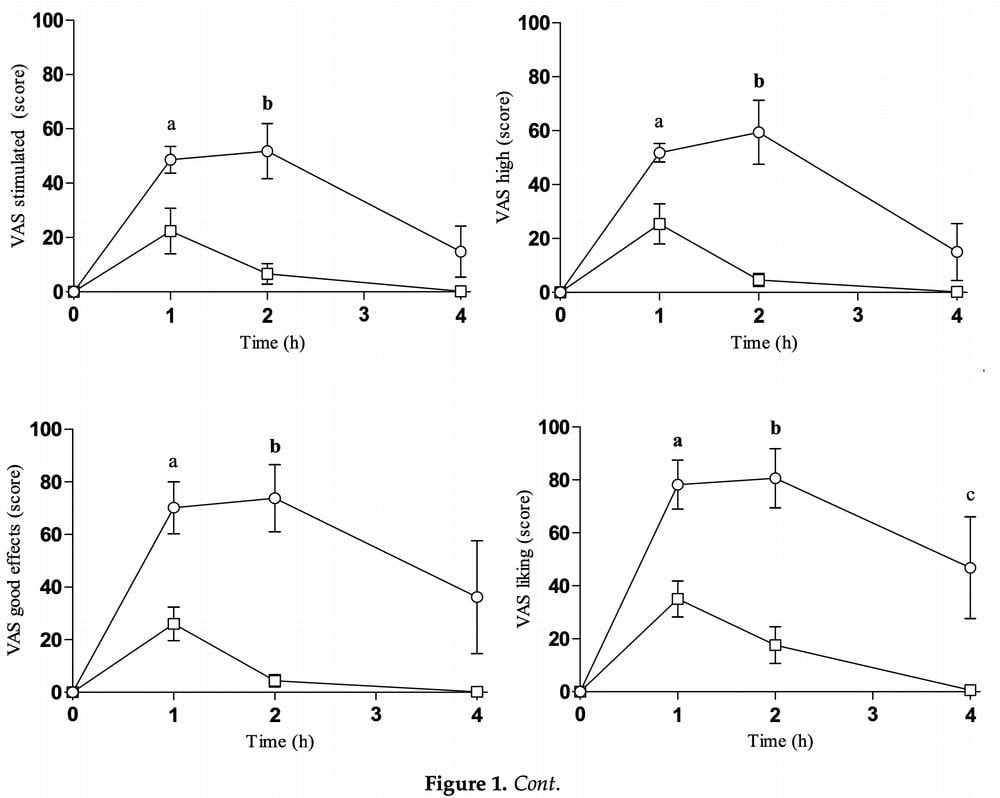
Given the ARCI scale data, mephedrone used both by nose and by mouth caused an increase in all assessed subscales. However, the most significant difference was an increase in the MBG (euphoria) and BG (mental performance and energy) subscales.
Statistically significant differences between modes of use were found only for the MBG subscale in the Emax and AUC scores. When the VESSPA-SEE questionnaire was examined, mephedrone showed increases on all subscales regardless of route of administration except the CP subscale (changes in perception) when mephedrone was administered intranasally. Statistical differences were demonstrated in the Emax and AUC for the SOC (pleasure and sociability) and ACT (activity and energy) subscales and only in the AUC for the PS (psychotic symptoms) subscale.
Pharmacokinetic parameters
As can be seen from the graph and table, after self-administered mephedrone use, its
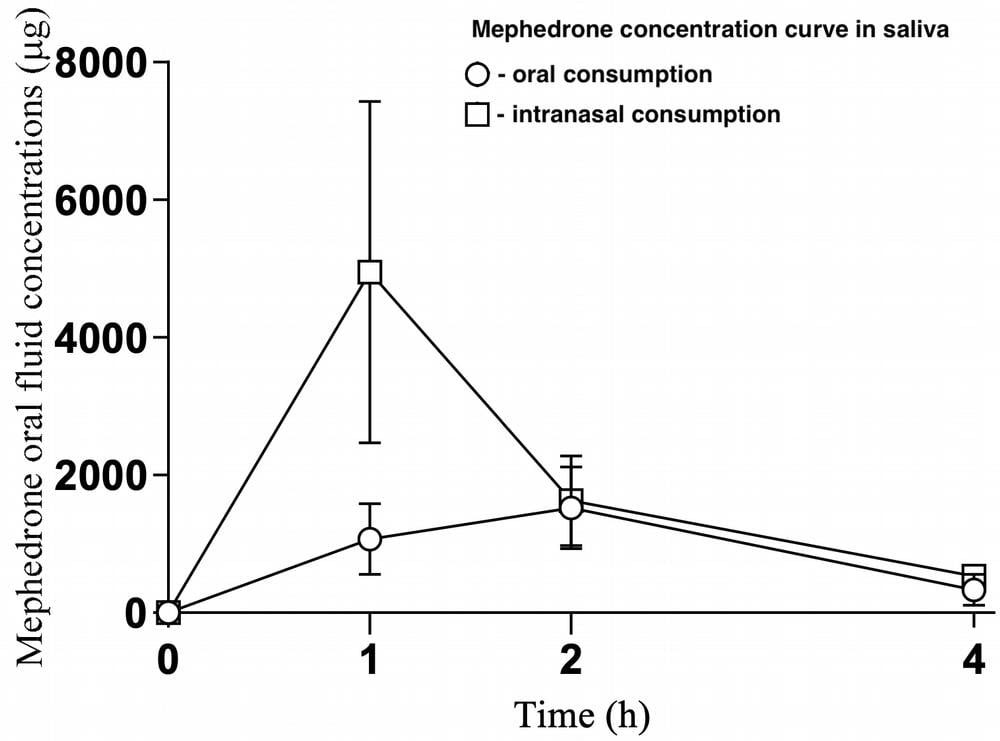
concentration increases rapidly, peaking two hours after ingestion, followed by a gradual decline over a period of four hours. The mean maximum concentration was about 1571 ng/mL, which was recorded two hours after drug ingestion. The AUC was 3686 ng-h/mL.
[table id=2 /]
When administered intranasally, the concentration peaks one hour after administration and then decreases rapidly.
The maximum concentration after one hour is 4950 ng/mL, after four hours the concentration of mephedrone is 9 times lower than the maximum concentration. The following are the metabolites found in urine 0-4 hours after use (demonstrated in more detail in Figure 2):
- Nor-mephedrone;
- Dihydro-mephedrone;
- 4-carboxy-mephedrone;
- Succinyl-nor-mephedrone in urine 0-4 hours after use.
Conclusion
The above results show that the self-administered use of mephedrone under natural observational conditions causes acute effects similar to those that occur under experimental conditions, as demonstrated to us by physiological objective and subjective indicators. When considering the pharmacological effects of oral use of mephedrone, the maximum intensity of its effects is reached later compared to intranasal use, and the severity of pharmacological effects is higher (including objective and subjective effects based on the ARCI and VESSPA scales). This is primarily due to the fact that when insufflated, mephedrone expands the vascular-rich areas of the intranasal cavity and pulmonary network, increasing the suction surface area and increasing the rate and volume of the substance entering the bloodstream.
In addition, the results obtained with respect to differences in mephedrone concentrations in oral fluid, as well as overall mephedrone and its metabolites, confirmed the fact that mephedrone concentrations were significantly higher following intranasal use compared with oral use. Given the mild intoxication cases analyzed, mephedrone concentrations in the urine were within the range of values measured in the study. Preliminary data from the present observational experiment indicate that the profiles of mephedrone under real-world conditions differ significantly depending on the route of administration, interindividual differences in pharmacodynamics and pharmacokinetics.
