
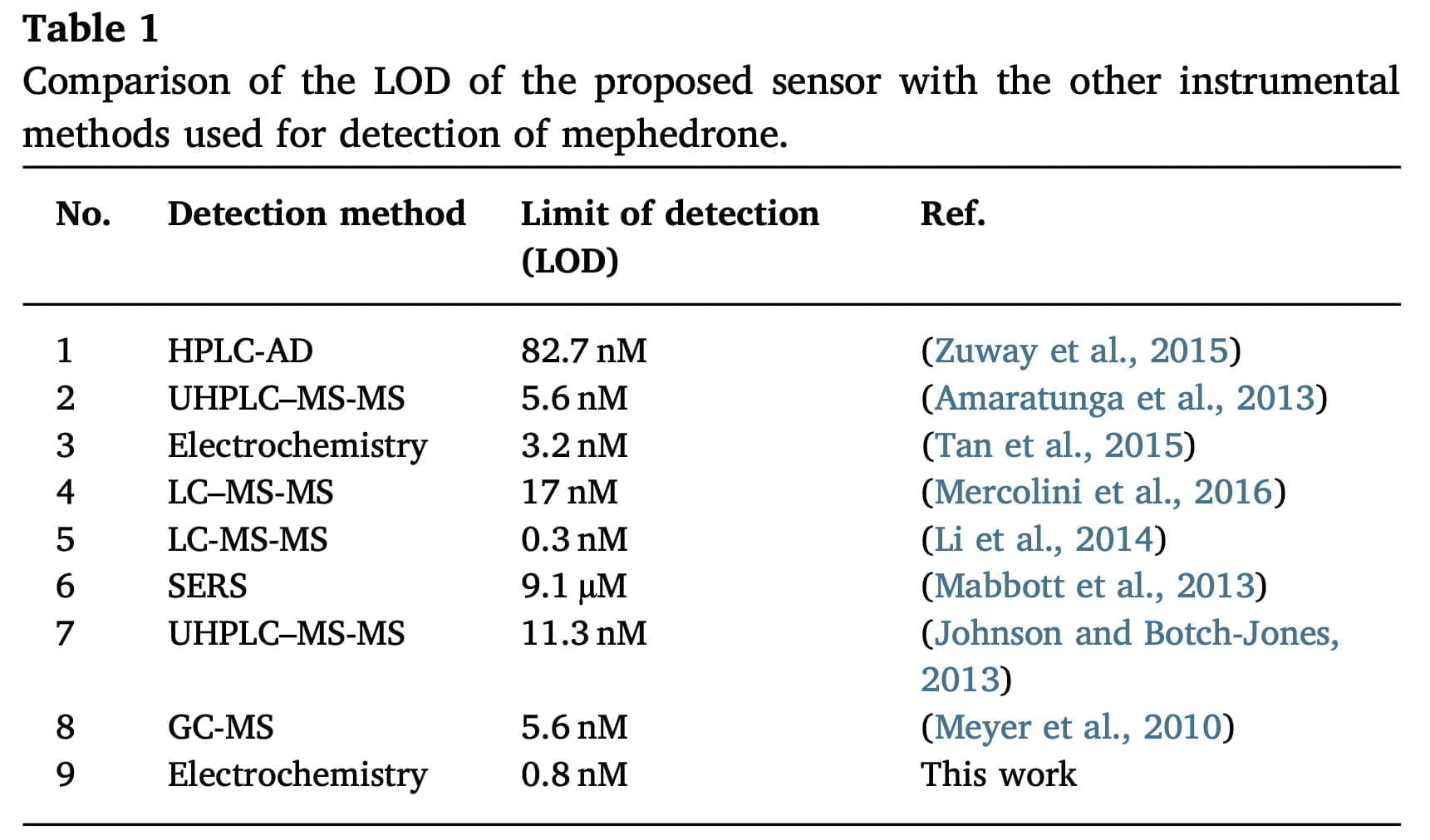
Many different analytical methods have been developed for the detection of mephedrone, such as identification by gas chromatography-mass spectrometry, high-performance liquid chromatography, and liquid chromatography with tandem mass spectrometry. Despite the numerous ways to detect substances, these methods are very time-consuming, expensive equipment, and specialists with sophisticated instruments. However, on the other hand, electrochemical methods for identifying substances are very simple, quite sensitive, and much less expensive than the above-mentioned methods.
A strong method that is frequently used to prepare polymeric material for molecular recognition is molecular imprinting. Due to its low cost, simplicity of synthesis, and great selectivity, the Molecular Imprinted Polymer (MIP) technique has been widely exploited in the development of numerous chemical sensors. However, the traditional MIP technique has been plagued by a number of drawbacks, including a delayed diffusion of the target away from the binding sites, a slow response time, a small surface area, and heterogeneous nature of the binding sites. As a result, some fresh methods for creating imprinting films have been discovered. One such procedure is the sol-gel imprinting. Sol-gel MIP provides certain notable advantages over conventional MIP technology, including as high porosity, thermal stability, ease of preparation, mild reaction conditions, and strong physical stiffness. To build chemical sensors, it is a good idea to combine molecular imprinting and the sol-gel method.

The development of various sensors has recently seen a large increase in the usage of composite materials based on the coupling of metal nanoparticles and CNTs with the aim of increasing the electrochemical and mechanical properties, which are not seen in the individual components. The desirable fusion of CNT properties, such as large surface area, high electrical conductivity, and chemical stability with good biocompatibility and unique electronic and optical properties of the gold nanoparticles results in a nanocomposite of CNTs and gold nanoparticles, which is of great interest due to the superior performance of the resulting sensing devices. One of the phenol derivatives, tyramine, has recently been used to develop various biosensors. If it is electro-polymerized through a phenolic compound, it easily forms a sticking film on the electrode surface. This publication describes the methodology of the first developed electrochemical MIP-sensor for the determination of mephedrone based on sol-gel using our technology as well as polythiramine and a nanocomposite. Using these materials in combination, we created a highly sensitive sensor with analytical characteristics for the quantitative determination of mephedrone in solutions.
Materials and methods used in our development
Mephedrone, whose purity was confirmed by chromatographic mass spectrometry, was used as the main test substance. Other consumable chemical reagents used were tetraethoxysilane (TEOS); phenyl- triethoxysilane (PTEOS); trifluoroaceticacid (TFA); sodium dodecyl sulfate (SDS) and tetrakis (hydroxymethyl) phosphonium chloride (THPC). The MWCNT nanocomposite was synthesized using the Deiminiat methodology. The sol solution was obtained by mixing 75 μL PTEOS, 75 μL TEOS, 700 μL H2O, 1100 μL EtOH, 10 μL of TFA and mephedrone (2.0 mM) in a vial and it was stirred for 2 h. The resulting solution was sonicated for 10 min. Next, the GC electrode was immersed into the sol solution. The MIP film was deposited on the surface of the electrode using cyclic voltammetry at the potential range between −0.8 and 1.2 V. To extract the template from the polymeric matrix, the modified GCE was first dried at ambient temperature for two hours before being submerged in a methanol-acetic acid (9:1, v/v) solution for 20 minutes. Under the same experimental circumstances, a non-imprinted polymer (NIP) electrode was also made, but without the addition of the template molecule to the solution.
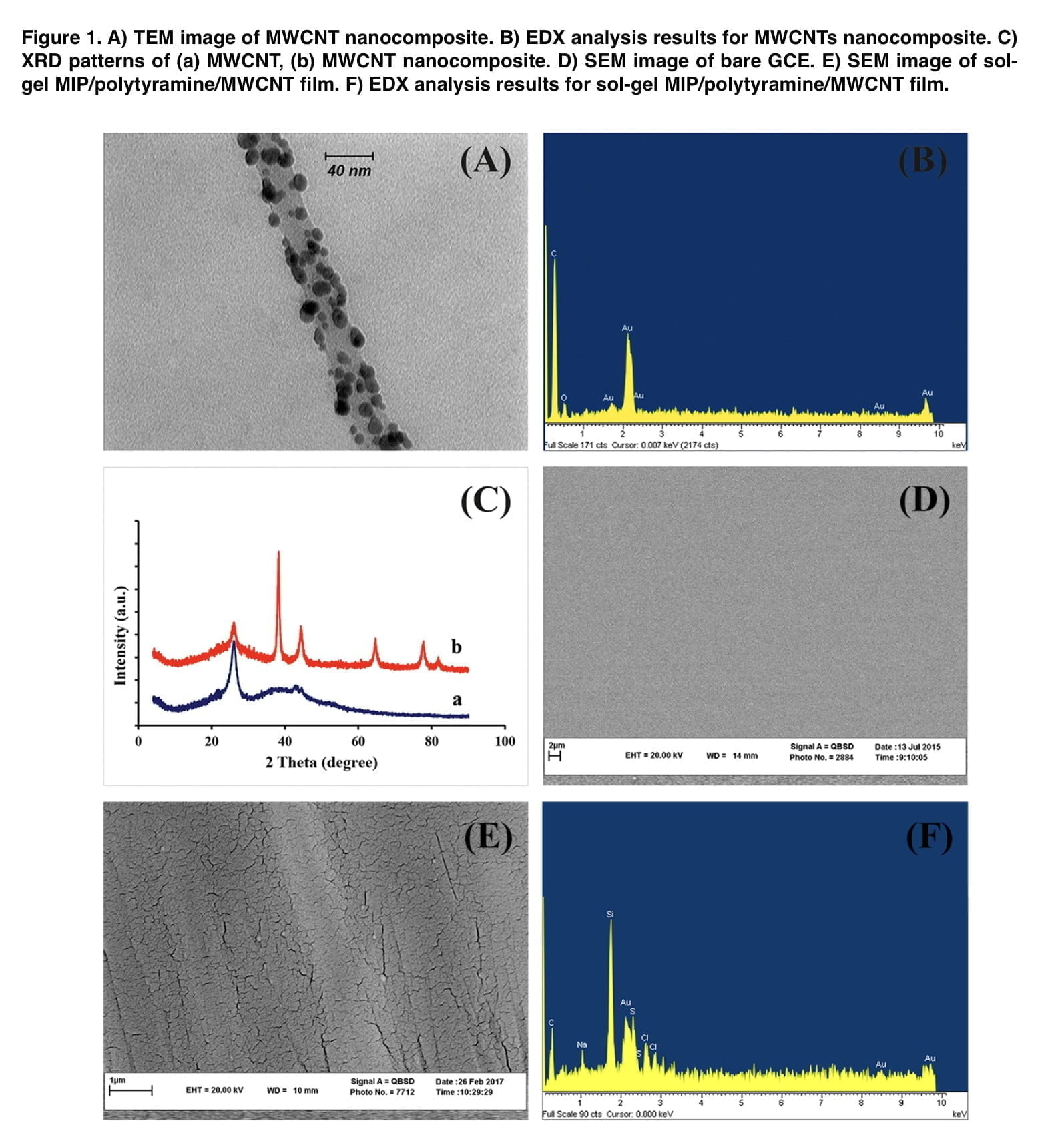
Discussing the results and drawing conclusions
The direct detection principle is based on removing the template from the polymer backing, after which voids are formed in it, which contribute to the diffusion of the active probe through the imprinted polymer to the electrode surface. Therefore, the concentration of mephedrone can be determined indirectly by measuring the intensity of the electrochemical signal of the redox probe. Fe(CN)6]3-/4 was used as a redox probe because mephedrone is not electroactive in the potential range under study. The number of imprinted cavities appears to be occupied in proportion to the increase in mephedrone concentration, which leads to a decrease in the current response. Δip was calculated by subtracting the current that was obtained in the absence of mephedrone from the current that was recorded in the presence of mephedrone molecules.
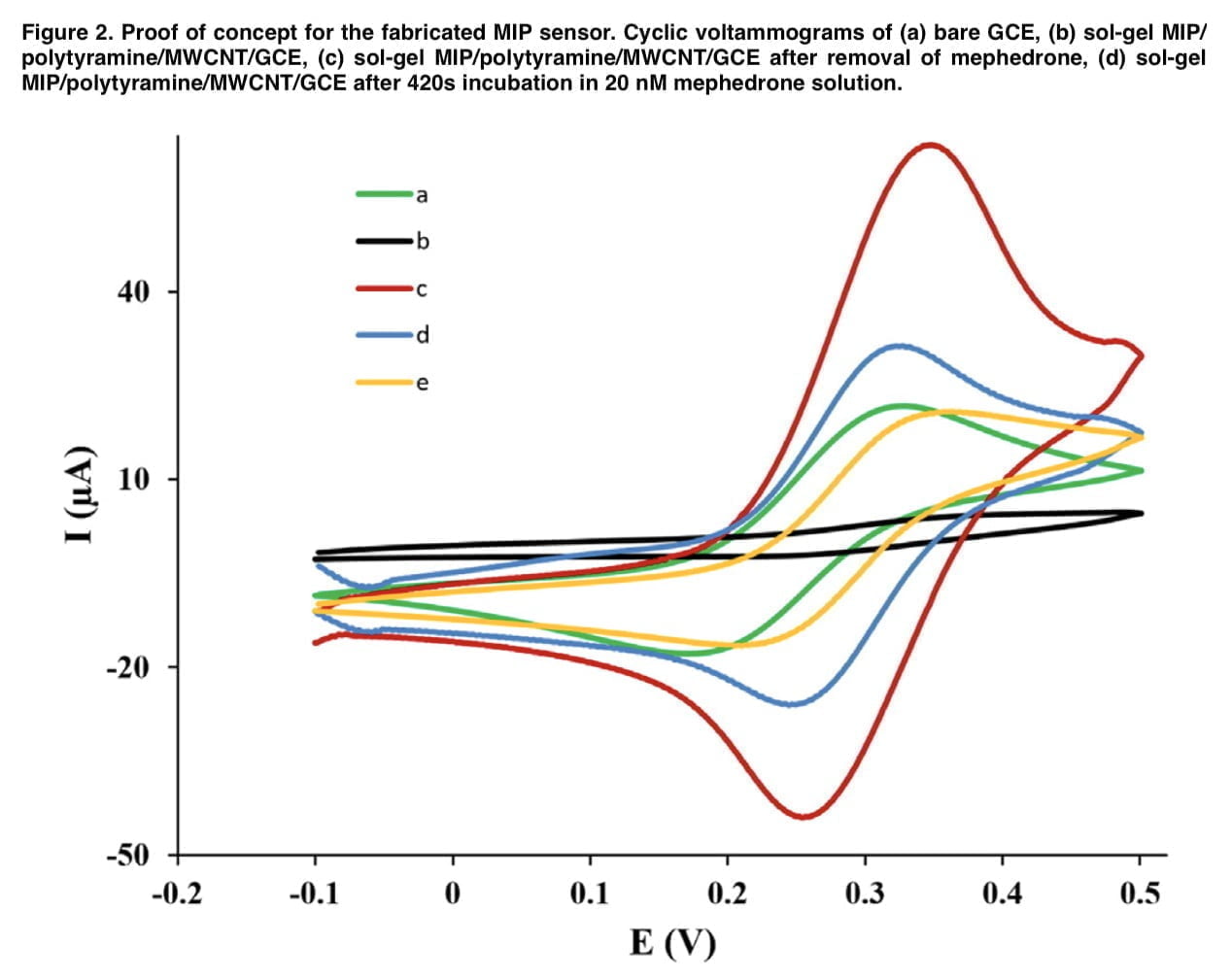
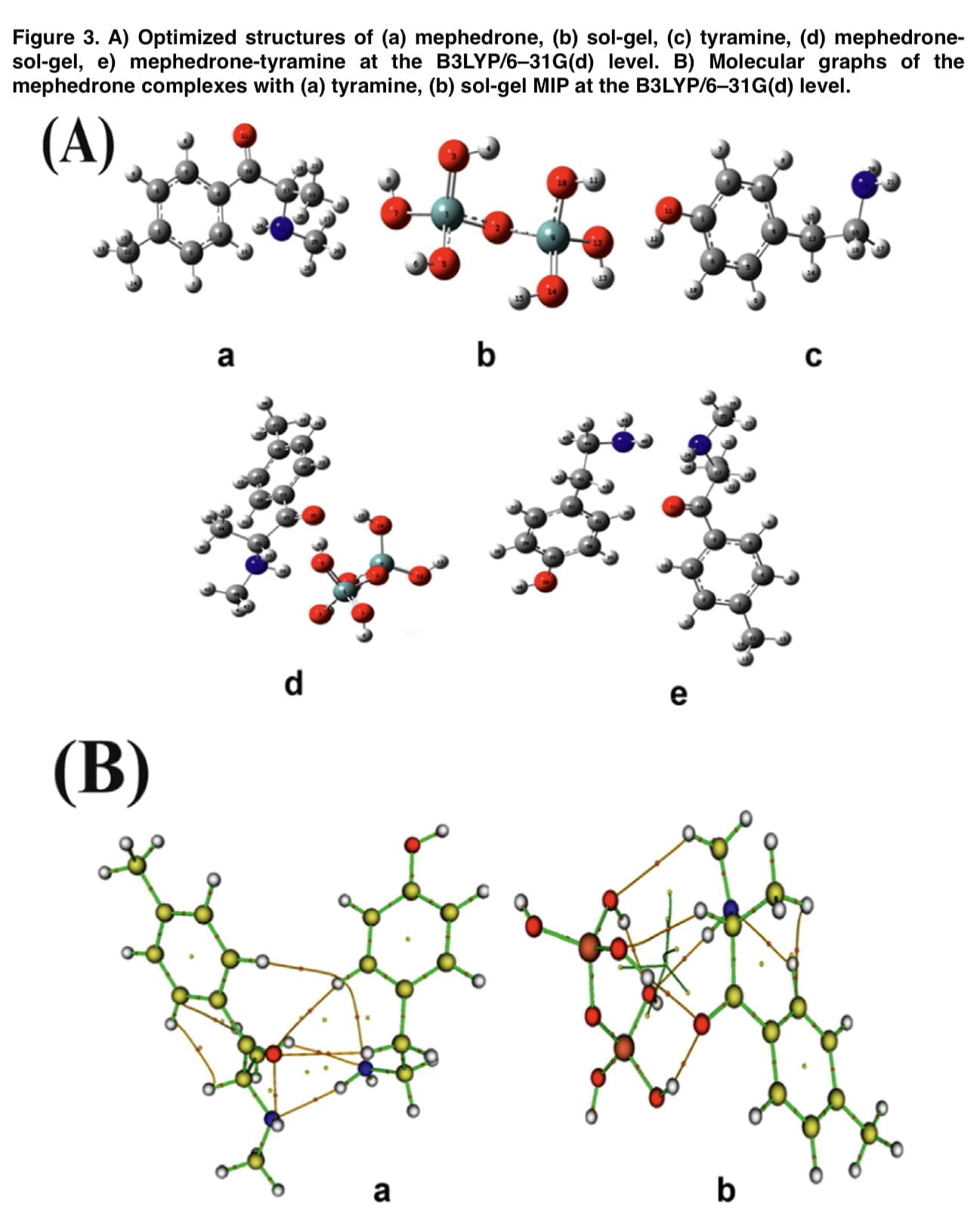
To determine the electrochemical properties of the sensor we performed cyclic voltammetric measurements. Figure 2 shows the CV profiles of the bare and modified electrodes in the presence of the redox probe. In the “e” curve, the interpretive response of the electrode decreases compared to the cyclic voltammetry diagram that was obtained for the MIP/polytyramine/MWCNT sol-gel. Such behavior shows that the presence of the nanocomposite in the matrix used greatly facilitates the electron transfer process, which is associated with higher conductivity. Figure 3(A) depicts the mephedrone, sol-gel MIP, and tyramine optimal structures as well as the mephedrone complexes with these substances. Mephedrone-tyramine and mephedrone-sol-gel MIP are predicted to interact with Gibbs free energies of 19.58 and 4.46 kJ mol-1, respectively. The mephedrone molecules interact with the tyramine molecule more strongly than the sol-gel MIP, according to the values of the Gibbs free energies. Figure 3 (B) shows the molecular plots of mephedrone complexes together with MIP sol-gel and tyramine. The results show that the calculated p-value for the H48-N23 indicators in the mephedrone-thiramine interaction is significantly higher than that for the O11-H43. The obtained above values of the positive laplacian show the electrostatic interaction between the mephedrone molecule and the functional monomers (demonstrated in Table 1). Several important factors, such as the number of scan cycles during the electropolymerization process, the pH of the mephedrone solution, and the incubation duration, were tuned in order to improve the analytical performance of the constructed electrochemical sensor.
A efficient electrochemical sensor should have a low detection limit, a wide concentration range for its target, and excellent selectivity toward a particular target. As a result, the findings of studying the impact of a few potentially interfering species found in bodily fluids, such as ascorbic acid, dopamine, and uric acid, on the detection of mephedrone are shown in Figure 4(C). A key parameter for investigation of the selectivity of the MIP sensor is imprinting factor, which is defined as the ratio of the ΔiMIP to the ΔiNIP. Also, Figure 4(C) shows an imprinting coefficient of 7.6 for mephedrone. However, the same parameter for ascorbic acid, dopamine, and uric acid is 1.2, 1.3, and 1.5, respectively. These experimental findings unequivocally support the proposed sensor’s greater selectivity for mephedrone molecules compared to competing species.
By determining the repeatability and reproducibility of the procedure, the accuracy of the electrochemical assays for measuring mephedrone in solution was assessed. Repeatable measurements of the mephedrone analyte with one of the manufactured imprinted sensors were used to obtain the repeatability, which is represented as the relative standard deviation (RSD). For this purpose, we measured the electrochemical response of the mephedrone solution for fourteen times, and the RSD was 3.6. These results prove that our developed molecular imprint sensor has extremely good reproducibility.
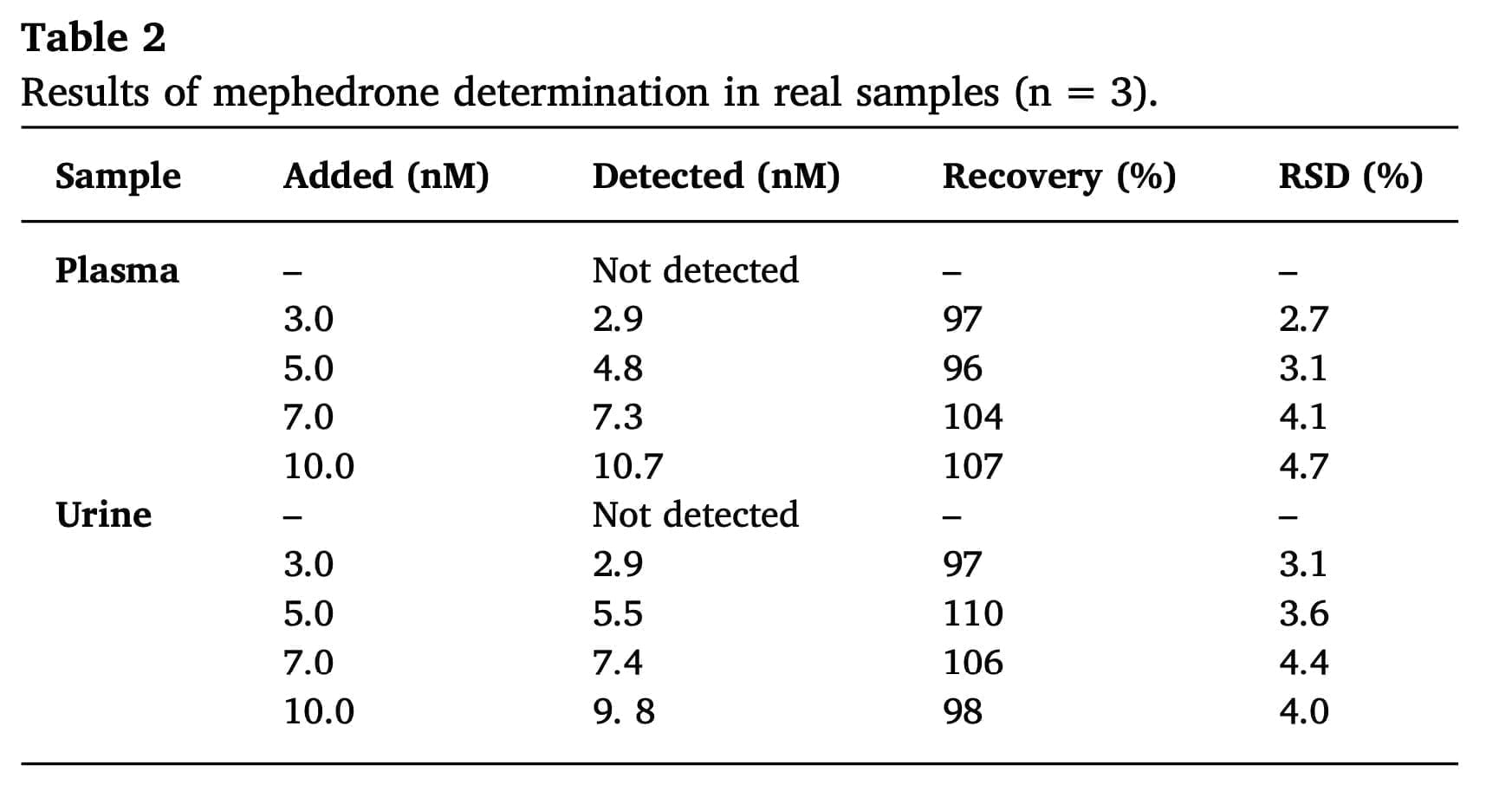
Even though the manufactured sensor demonstrated good merit values when utilizing mephedrone standard solutions, it is important to evaluate this sensor’s analytical performance using actual samples. In order to quantify mephedrone in samples of urine and plasma, which are biological fluids and contain a mixture of proteins and the other interfering compounds, the sensor was created. Table 2 provides an overview of the measures findings. Examining the observed results demonstrates that the suggested sensor for finding mephedrone in urine and plasma samples works as intended.
The electrochemical sensor developed by us for the determination of mephedrone in solutions makes it possible to realize ultra-sensitive selective identification of its molecules using low cost consumables and equipment. It overcomes some drawbacks of standard MIP-based sensors (e.g., weak electrochemical response, long response time, and complexity of sensor preparation). In addition, the sensor shows a wide detection limit and good stability, reproducibility and repeatability. The acceptable recovery rate shows that this sensor can be used for the future identification of mephedrone in biological samples in a routine methodology.
