Mephedrone metabolism has been studied in vitro and in vivo in both animal models and people in the past. We’ve previously written a number of pieces on our site on mephedrone’s metabolism. The secondary amine is N-demethylated, the ketone moiety is reduced to the hydroxyl group, and the tolyl moiety is oxidized to form the main phase I metabolites. It has been established that hepatic cytochrome P450 2D6 (CYP2D6) is the primary enzyme involved in the metabolism of mephedrone in humans, with other cytochrome P450 enzymes contributing very little.
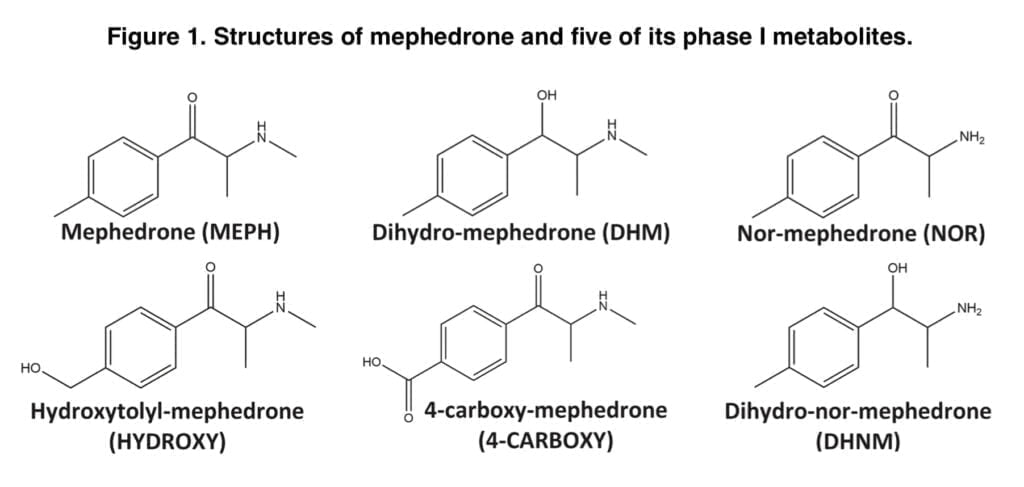
Mephedrone distribution in urine and some of its metabolites have only been studied in two controlled human administration trials to far. Mephedrone was consumed orally in both investigations, and samples were analyzed using either gas chromatography-mass spectrometry or liquid chromatography-tandem mass spectrometry. According to these research, 4-carboxy-mephedrone was the most prevalent metabolite, with concentrations that were nearly ten times greater than those of mephedrone itself. A limited urine recovery was also seen for mephedrone, with only around 1.15 percent of the entire given dosage recovered after LC-MS/MS analysis and 15.4 percent after GC-MS analysis. Olesti and colleagues have demonstrated that urine recovery of mephedrone and its metabolites is proportionate to the dosages provided. There is no information on the urine clearance of mephedrone and its metabolites following this method of administration as part of a controlled trial, despite the fact that nasal insufflation is probably the most popular way to use mephedrone. Here, we provide the findings of a research in which, following controlled nasal administration of 100 mg of pure mephedrone hydrochloride to six volunteers, urine samples were quantitatively analyzed for mephedrone and five of its phase I metabolites (Figure 1). Pharmacokinetic (PK) data discovered by examination of whole blood and plasma gathered for the same investigation have previously been presented.
Materials and methods used for this study
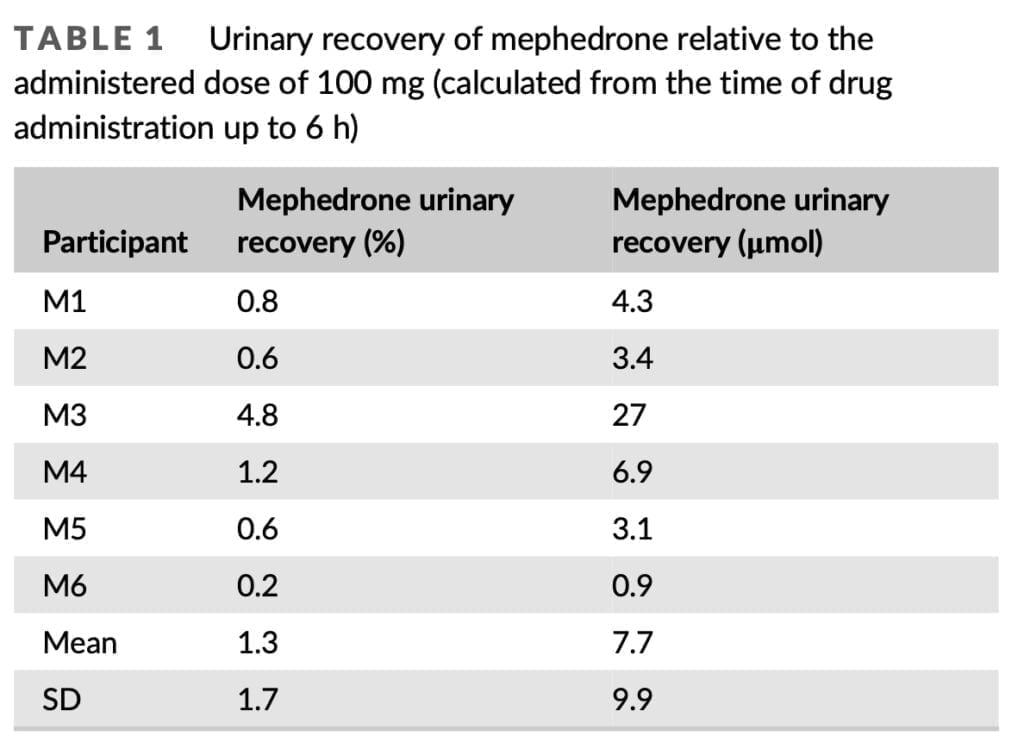
In our study, we used mephedrone hydrochloride as well as the independently synthesized metabolite dihydro-nor-mephedrone. The purity of the substances used was confirmed by high-resolution mass spectrometry and nuclear magnetic resonance analysis. Volunteers who were occasional users of mephedrone and/or other psychostimulants were included in the present study. Volunteers were monitored throughout the study and were free of any psychoactive substances for one week prior to taking mephedrone. Six healthy men consumed 100 mg of mephedrone hydrochloride (a racemic mixture of 96.3% purity) intranasally. Urine samples were collected at 0 and 6 hours after consumption and then on days 2, 3, and 30 of the study. A modified solid phase extraction (SPE) method developed for the extraction of mephedrone and its metabolites from human plasma (published elsewhere) was used. Briefly, 250 μl of urine was extracted. Where dilution was required, samples were diluted 1 in 100 or 1 in 1000 in the blank matrix alongside three additional QCs prepared in the same manner. After SPE, samples were dried under nitrogen and were reconstituted with 100 μl of 0.1% formic acid in acetonitrile. Total mephedrone eliminated in urine was calculated for each participant by multiplying mephedrone concentration in each urine sample by the urinary volume collected at each timepoint. For each participant, the total amount of mephedrone removed in urine was estimated by multiplying the mephedrone concentration in each sample of urine by the amount of pee that was taken at each timepoint. By dividing the total quantity of mephedrone discharged in urine by the previously reported area under the plasma concentration-time curve, renal clearance was estimated.
Analysis and discussion of the results
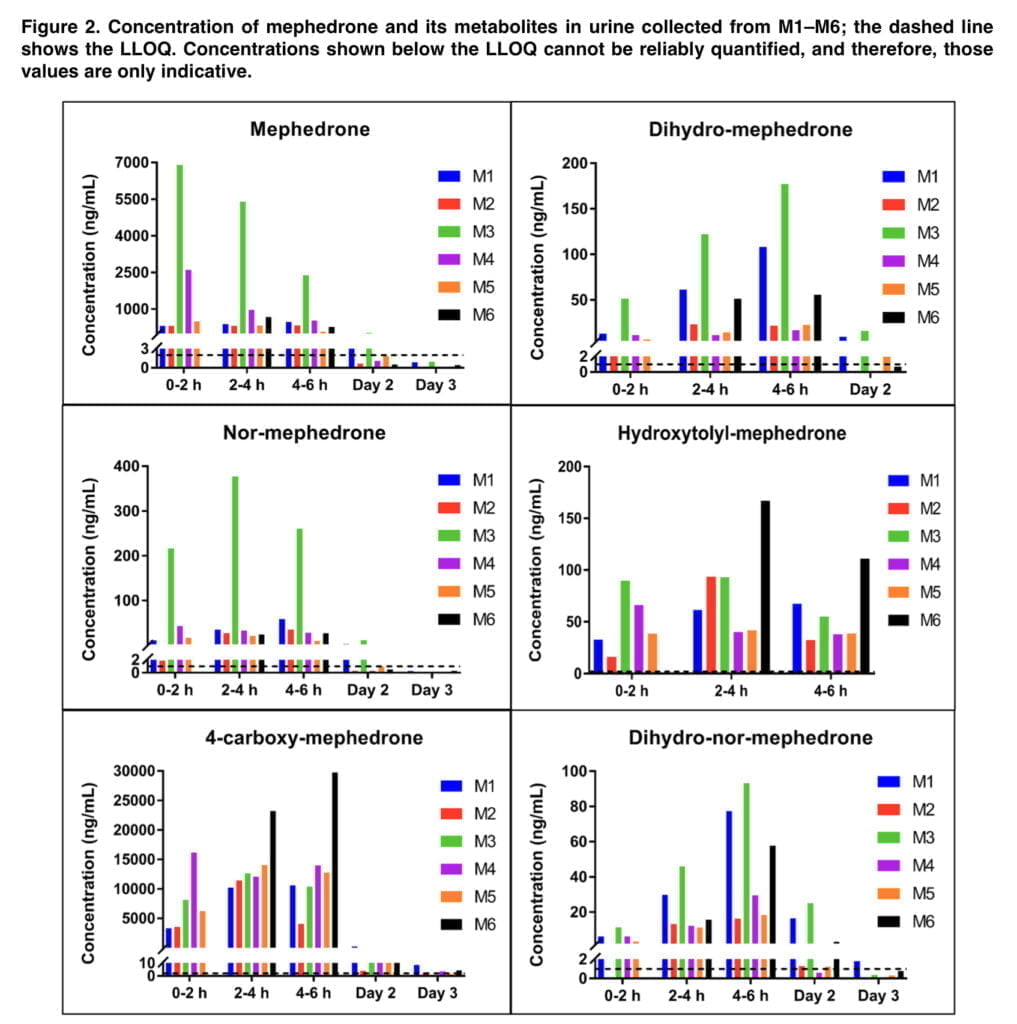
Figure 2 displays the levels of mephedrone and its metabolites in urine taken from six people (referred to as M1-M6). Analytes were not found at Day 2 or Day 3 timepoints when Day 2 or Day 3 data is not displayed. Because participant 6 was unable to provide a urine sample within the first two hours of mephedrone use, there are no indicators of this time period in the above figure. Urine samples taken 30 days after treatment and pre-administration did not contain any analyzers. 4-carboxy reached the highest concentrations in urine (Cmax = 29.8 μg/ml), followed by mephedrone (Cmax = 6.98 μg/ml) and NOR (Cmax = 377 ng/ml). DHM (Cmax = 177 ng/ml) and HYDROXY (Cmax = 167 ng/ml) were detected at lower concentrations, whereas DHNM was found at the lowest concentrations (Cmax = 93.1 ng/ml). The greatest levels of mephedrone, DHM, NOR, and DHNM between 0 and 6 hours were seen in participant M3, who had significantly greater levels of mephedrone and NOR than the other subjects.
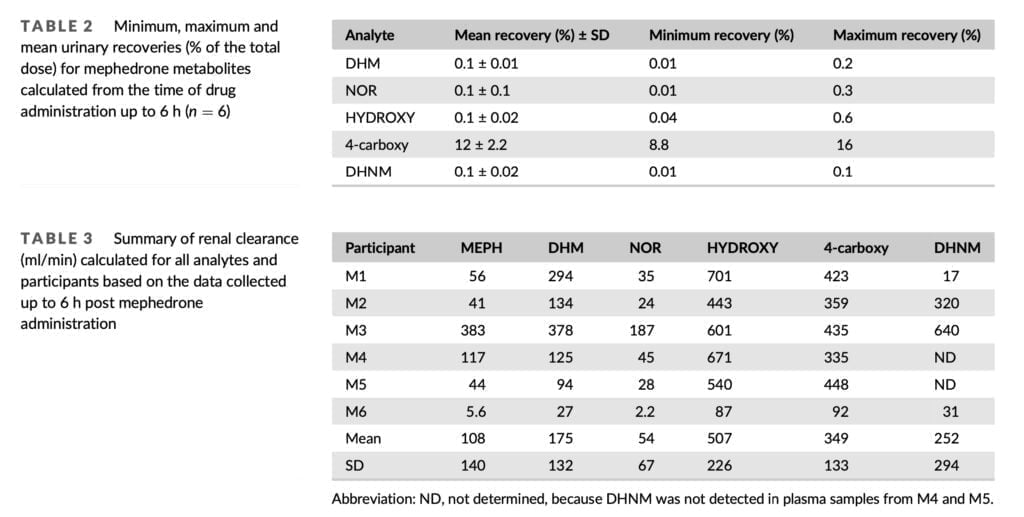
We detected 4-carboxy and DHNM in urine even on the third day, so the detection window for mephedrone can be extended by at least 48 hours. DHM, NOR, and 4-carboxy were found in urine up to 48 hours after 150 mg of mephedrone was administered orally to six healthy male volunteers in the prior investigation. From the standpoint of forensic casework, the ability to identify mephedrone metabolites up to three days after injection provides an invaluable tool. When mephedrone is detected in urine at or close to its limit of detection, the presence of 4-carboxy and/or DHNM provides further evidence that the substance has been given. A variety of phase II metabolites of mephedrone have been identified in the past, therefore looking for their existence has similar evidentiary value. Our excretion investigation was limited to the analysis of mephedrone and its phase I metabolites since it was focused on quantification and because phase II standards were not readily accessible in the marketplace. This remains the case for conjugates of metabolites of many new psychoactive substances because synthesis is often challenging and expensive, and commercially not viable to undertake in anticipation of limited demand for such products.
Only 1.3% of the 100 mg total given dosage of mephedrone was found in urine after 6 hours. That is extremely similar to a total urinary recovery estimate of 1.2% that was previously published. It was based on the examination of urine samples that were continually collected for 48 hours after oral administration of 150 mg of mephedrone hydrochloride. Small levels of mephedrone were likely still secreted during the 6 h timepoint on Days 1 and 2 of our investigation, but urine samples were not taken during that time. The 1.3% urinary recovery does not, therefore, reflect the whole urine recovery. With a mean renal clearance of 108 ml/min, mephedrone was swiftly removed from the body. This is consistent with the previously reported renal clearance for mephedrone of 5.6 L/h, which was determined by analyzing urine samples collected over a 24-hour period after oral administration of 150 mg of mephedrone hydrochloride.
Despite the fact that M3’s PK blood profile was comparable to that of M1-M5, far greater levels of mephedrone and NOR were found in the urine samples taken from this person. Additionally, M3 had mephedrone recoveries that were nearly an order of magnitude larger than those of the other subjects. M6, on the other hand, exhibited the lowest renal clearance while having a noticeable change in the PK profile in blood. With a mean renal clearance of 108 ml/min, mephedrone was swiftly removed from the body. This is consistent with the previously reported renal clearance for mephedrone of 5.6 L/h, which was determined by analyzing urine samples collected over a 24-hour period after oral administration of 150 mg of mephedrone hydrochloride.
Conclusion
Mephedrone and five of its phase I metabolites may be quantified simultaneously in human urine using a technology that has been extensively verified. This approach was used to analyze urine samples from healthy individuals who participated in a controlled administration research. All analytes were found in the urine after intranasal insufflation, with 4-carboxy having the greatest concentration. The only metabolites found in all urine samples on Day 3 post-administration were 4-carboxy and DHNM, increasing the period of time during which mephedrone usage could be detected.
