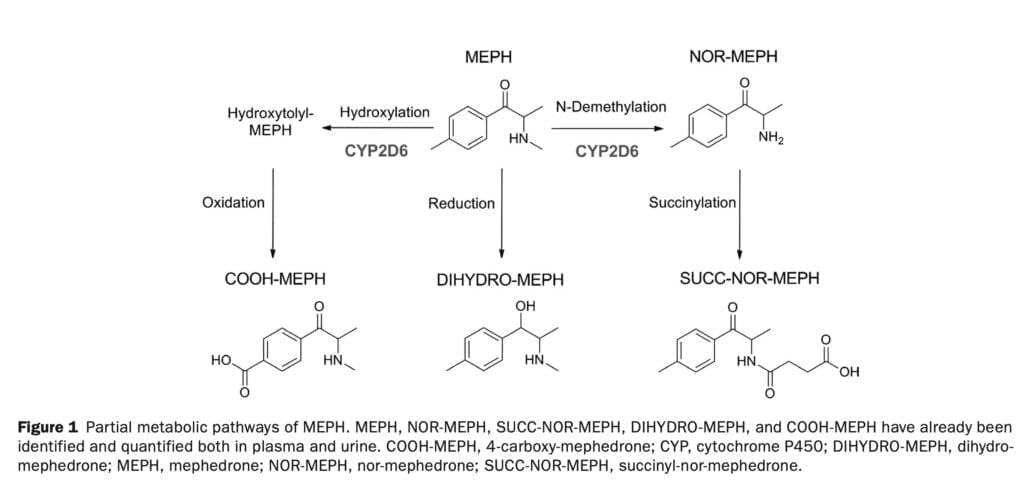
When compared to 3,4-Methylenedioxymethamphetamine, mephedrone has a quicker start and shorter duration, but still exhibits the physiological and subjective effects (such as increased arterial blood pressure, heart rate, pupil diameter, and body temperature) typical of a psychostimulant substance. Mephedrone’s metabolic disposition in animal models is characterized by limited oral bioavailability and a significant amount of hepatic metabolism. Its metabolism has been described in rats, liver hepatocytes, human liver microsomes, and human biological samples. N-demethylation, hydroxylation, oxidation, reduction, and conjugation with carboxylic acids and glucuronides are the main metabolic processes involved in the liver’s metabolism of mephedrone (Figure 1). The cytochrome P450 2D6 (CYP2D6), a highly polymorphic drug metabolizing enzyme, has been described in vitro as mephedrone with some other contributions from NAPDH-dependent enzymes. Other synthetic cathinones’ metabolism has also been linked to CYP2D6 (such as methylone). At this time, there is no generalized and complete information on the effect of this cytochrome polymorphism on human uptake of mephedrone, and there is no information on the possible therapeutic effects of this polymorphism.
Recently, our research team conducted a study of pharmacokinetic parameters of mephedrone after taking 150 mg of the substance, the study analyzed the parameters of the following metabolites: nor-mephedrone, dihydro-mephedrone, 4-carboxy-mephedrone and succinyl-nor-mephedrone. It is currently unknown, however, whether the recovery of metabolites varies in a dose-dependent manner. Mephedrone oral dosages have been reported to range from 15 to 250 mg, and it is uncertain if, like MDMA, MEPH pharmacokinetics is non linear. Although the pharmacokinetics of mephedrone have been studied in animal models using a bicompartmental model, studies on various doses in humans are still being conducted.
In the present publication, we describe a study in which a dose-dependent pharmacological analysis of mephedrone and its metabolites was performed in healthy subjects who took different doses of the substance. The resulting concentrations of the main substance and its metabolites were used to describe pharmacokinetics. Mephedrone’s pharmacokinetics have been described using information on the amounts of the drug and its metabolites in plasma and urine. Additionally, after the administration of mephedrone doses, serotonin (5-HT) concentrations in human blood were monitored and correlated with mephedrone and metabolite concentrations as well as the subjects’ CYP2D6 phenotype. Pharmacodynamic parameters (cardiovascular and subjective effects) were also assessed.
Materials used and design of the experiment
In the first phase of our experiment, we recruited nine healthy men who participated in the first phase of a double-blind clinical trial. Volunteers were given one oral dose of mephedrone in one session, which was separated by one week of washout. Of the nine men, three took 50 and 100 mg of mephedrone, and the rest took 150 and 200 mg. Amphetamines, ecstasy, mephedrone, and other cathinones were used recreationally by the subjects. Participants ranged in age from 34 to 41 and weight from 63 to 73 kg. Following the current pilot trial, participants took part in a clinical investigation in which 200 mg of mephedrone was administered. Subjects were phenotyped with dextromethorphan, and only those with a dextromethorphan/dextrorphan ratio >0.3 were included in the study. Additionally, CYP2D6 genotyping was performed using the PHARMAchip DNA array. Following the study of the subjects’ genotypes, phenotypes were given to the volunteer participants, and the activity score for CYP2D6 was determined for each subject. During the experiment, blood samples were collected before the injection of the substance, as well as 1-2-4-6 and 8 hours after the administration of mephedrone. Urine samples were collected at intervals of 4, 6, 12 and 24 hours.
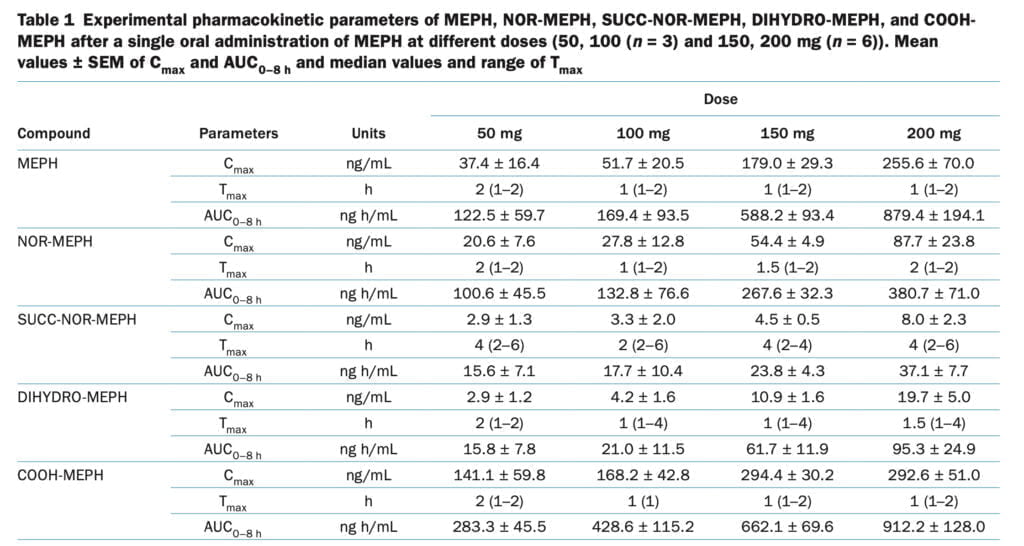
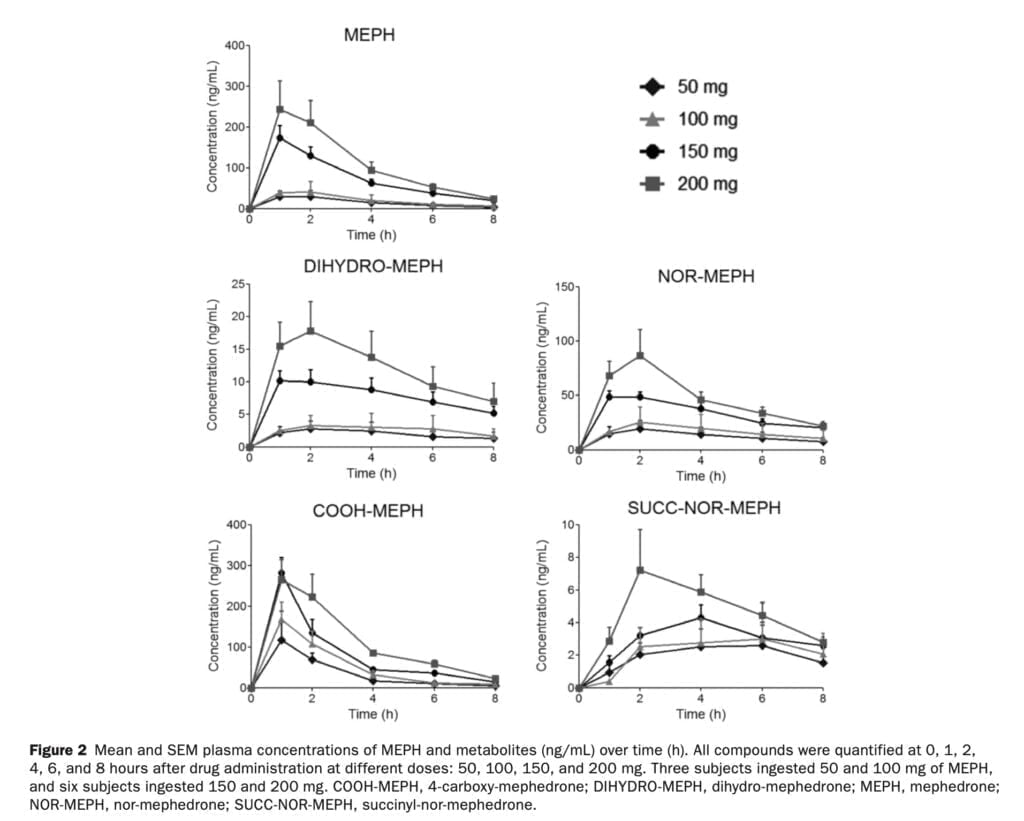
The main pharmacokinetic parameters analyzed were Tmax, AUC (0-8 h), T1/2, bioavailability, and apparent CLp (Figure 1). To study pharmacodynamic parameters we used blood pressure and heart rate, which were assessed at baseline, as well as 1-2-4-6 and 8 hours after the use of mephedrone. Subjective effects were evaluated using 100-mm visual analogue scales labeled with different adjectives (such as stimulant, high, or good effects). Subjects were asked to score effects they were experiencing at different time points after drug administration. Tandem mass spectrometry was used as an analytical method to identify mephedrone metabolites. To estimate the concentration of free serotonin in blood, we used high-performance liquid chromatography with electrochemical detection.
Discussion of the results
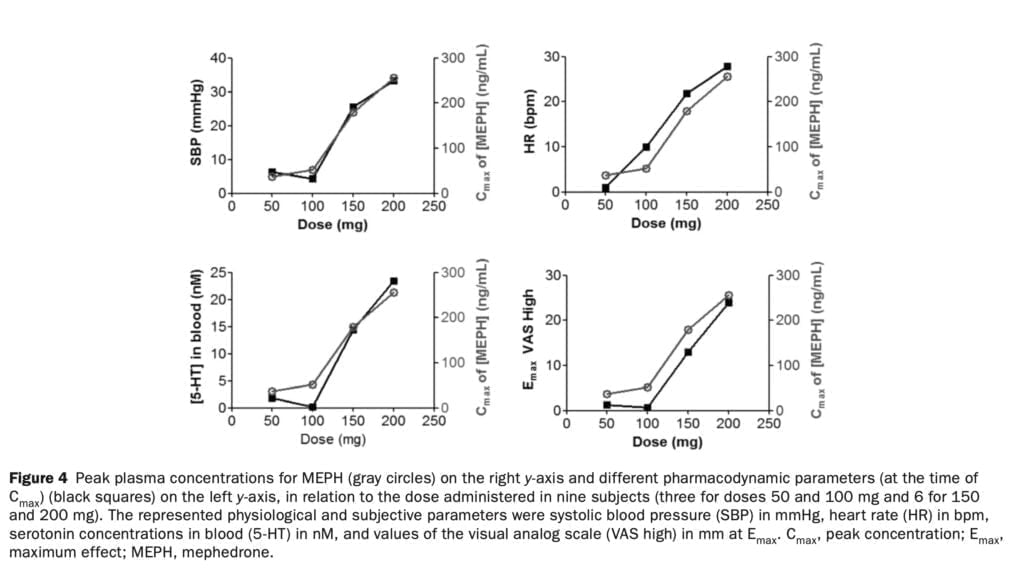
Prior to doing a bigger investigation to examine the clinical pharmacology of mephedrone and MDMA, we tested four dosages of the drug in people in a pilot dose-finding trial. We describe for the first time a dose-dependent relationship between mephedrone plasma concentrations, physiological and subjective effects, and serotonin blood levels in people. We also describe for the first time that MEPH metabolic disposition in humans is impacted by CYP2D6 allelic variation. Regarding pharmacodynamic effects, we discovered a linear relationship between mephedrone concentrations (Cmax) and the outcomes (Emax) they produced. We did not, however, observe a correlation between mephedrone metabolites, including NOR-MEPH, which is reportedly pharmacologically active and the monitored pharmacodynamic effects. The results for subjective (VAS high, good effects, stimulated) and cardiovascular (SBP and HR) effects were positively correlated with drug concentrations in plasma at each mephedrone dosage examined (Figure 4).
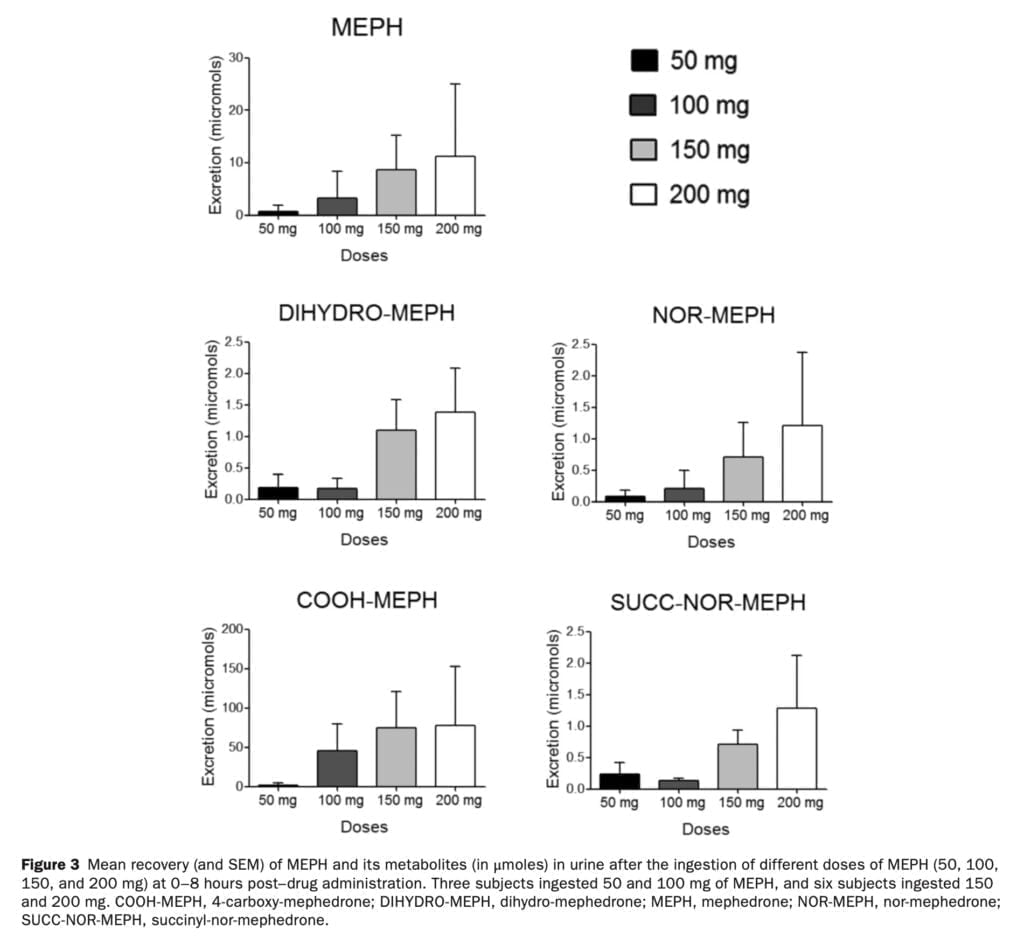
In the rat brain, mephedrone, like MDMA, causes a fast release and removal of serotonin. We have previously discussed how MDMA increases the gene expression of the serotonin transporter in blood platelets in humans. In the current study, we found that the release of serotonin from platelets following mephedrone injection was dose-dependent. Similar to the rise in 5-HT following MDMA delivery, 5-HT concentrations peaked an hour after mephedrone injection. The pharmacokinetics of mephedrone and its metabolites was investigated after the administration of four different doses. Mephedrone was rapidly absorbed at all doses, plasma concentrations peaked at around 1 hour post–drug administration, and the elimination process was rapid, in accordance with previous studies. For all examined dosages, the profile of metabolites recovered in urine was consistent. Mephedrone’s weak and erratic oral bioavailability in rats, which is about six times lower than that of MDMA, and its normal metabolism, which is largely regulated by CYP2D6, are likely factors in the drug’s substantial inter-individual variability in recovery. Despite the obvious variations in the pharmacokinetics of mephedrone, the data indicate that, in contrast to MDMA, its kinetics is linear within the investigated dosage range (50-200 mg). At larger dosages of 4-MMC that are unlikely to be consumed by people unless there is acute intoxication, rats showed non linear pharmacokinetic behavior.
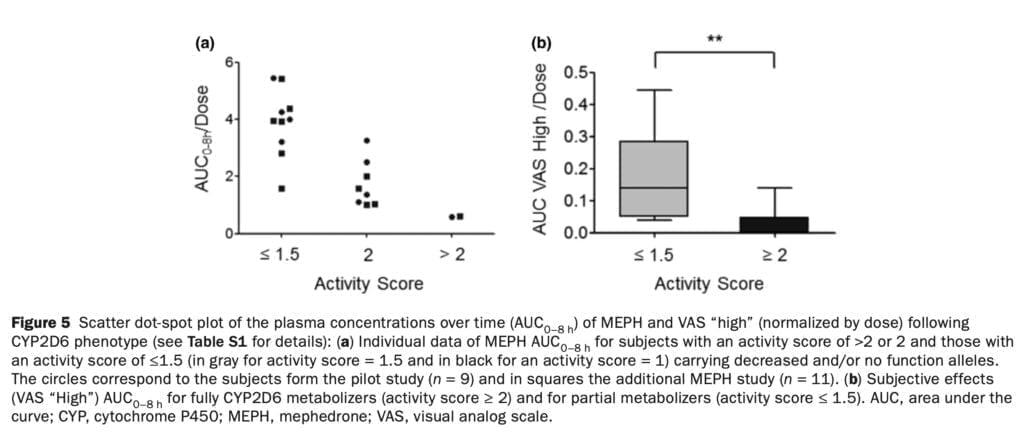
Studies conducted in vitro indicate that CYP2D6 in the human liver is responsible for the metabolic disposition of the majority of amphetamine analogs and other psychostimulant medications. Similar to this, in vitro research has indicated that CYP2D6 is essential for the metabolism of 4-MMC. We discovered that CYP2D6 activity considerably changed the plasma concentrations of mephedrone and its pharmacodynamic impact in our investigation (Figure 5). Such a discovery emphasizes the importance of the unique CYP2D6 genotype/phenotype: ultrarapid metabolizers are anticipated to require more frequent dosing to achieve the desired effects, whereas poor metabolizers are anticipated to have greater concentrations and stronger 4-MMC effects. Additionally, blood levels of mephedrone and 3-methylmethcathinone in fatal intoxications were greater than in non-fatal instances, which may have been influenced by CYP2D6 genetic variation.
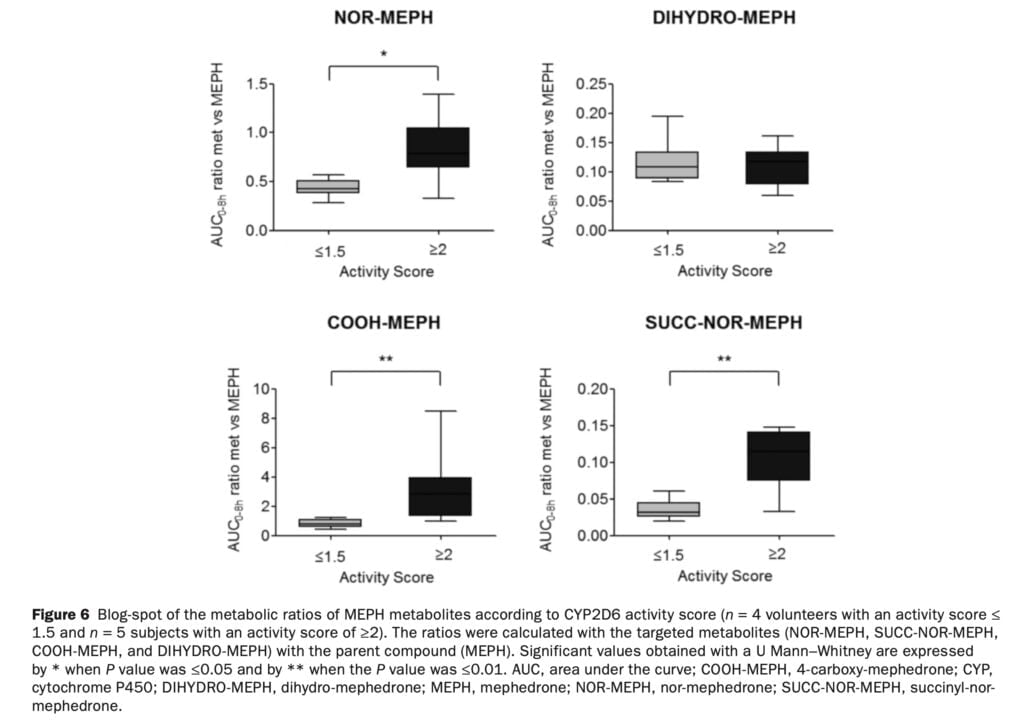
Hydroxytolyl-MEPH could not be measured in biological fluids because of a lack of reference materials. Nevertheless, the fact that the COOH-MEPH:MEPH ratio is modulated by CYP2D6 (Figure 6) is most likely due to the availability of its precursor hydroxytolyl-MEPH. The computed metabolite/parent compound ratios supported the idea that CYP2D6 was involved in the first two metabolic stages that produced NOR-MEPH and hydroxytolyl-MEPH (Figure 1).
Conclusion
The contribution of other enzymes to the metabolite formation cannot be ruled out. It appears, however, that both main pathways are primarily mediated by CYP2D6; of particular importance is the one leading to hydroxytolyl-MEPH and COOH-MEPH formation, the most relevant quantitatively in humans. Concerning DIHYDRO-MEPH, it appears that CYP2D6 was not involved in its formation as no alterations of the DIHYDRO-MEPH:MEPH ratio were found. Since CYP2D6 genotype can, at least in part, account for MEPH accumulation in the body, genetic variation appears to have a role in the wide interindividual variance in clinical toxicity related to MEPH usage. The effects related to CYP2D6 may be obscured by the significant variation in 4-MMC bioavailability, hence its importance in situations of acute intoxication should be reduced. Information would be useful if it were evaluated with a bigger sample size of female participants and alternative delivery methods (such as insufflation). Furthermore, accurate quantification was impossible because other metabolites lacked analytical standards. Studies on the pharmacological action of the 4-MMC metabolites and their role in negative effects would need to be conducted with a bigger population. However, our investigation has enabled a comprehensive assessment of the pharmacokinetic, pharmacodynamic, and pharmacogenetic clinical pharmacology of mephedrone at a range of dosages relevant to recreational users.
