Based on the experimental data obtained, it was found that exposure to mephedrone leads to a decrease in mitochondrial biogenesis and induced mitochondrial dysfunction and toxicity. In general, the mitochondrial cycle implements energy production and also contributes greatly to the regulation of calcium signals, redox status, and programmed cell death. Mitochondria are one of the main targets of bioenergetic insufficiency induced by xenobiotics. Mitochondrial dysfunction leads to cell death due to impaired ATP production and dysregulation of calcium metabolism. One important aspect of evaluating the safety of various chemicals is the assessment of their potential devolopmental and reproductive toxicity. In this publication, we describe a study that we performed on the mitochondrial and histopathological assessment of the embryo-fetal toxicity of mephedrone in mice.
Materials and methods of research
In our study, we used the following substances and reagents: xylazine; ketamine; bovine serum albumin (BSA); N-(2-hydroxyethyl) pi-perazine-N0-(2-ethanesul-fonic acid) (HEPES); dimethyl sulfoxide (DMSO); 2-Amino-2- hydroxymethyl-propane-1,3-diol (TRIS),; monopotassium phosphate; 4,5-dimethylthiazol-2-yl)-2,5-diphenyltetra- zolium bromide (MTT), etc. Pregnant female mice injected intraperitoneally with mephedrone in doses: 5, 10, 20 and 50 mg/kg were used in the experiment. After anesthesia on the 15th day of pregnancy, a laparotomy was performed with uterine exteriorization to identify the location and number of fetuses, measure length and weight, then the fetus was stained with hematoxylin-eosin and stereomicroscopy was performed to verify the presence of malformations. After embryo separation, the brain, liver, heart, and placenta were isolated from the mice, which were separately homogenized using a glass hand homogenizer in ice-cold medium to isolate mitochondria. The integrity and purity of the mitochondria were verified by SDH and lactate dehydrogenase assays. In order to quantify the production of mitochondrial reactive oxygen species, we used a fluorescence spectrophotometer. Rhodamine 123 has been used as a cationic fluorescent dye to determine the mitochondrial membrane potential (MMP).
Obtained results, their analysis and discussion
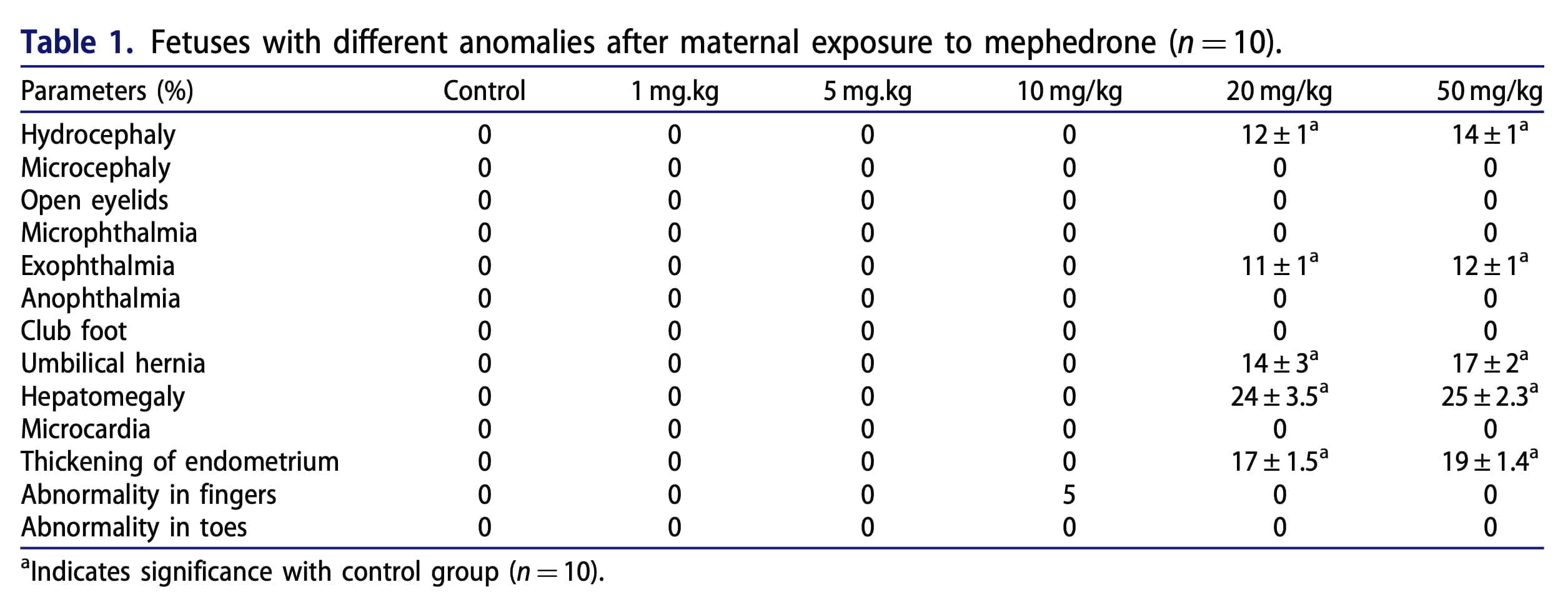
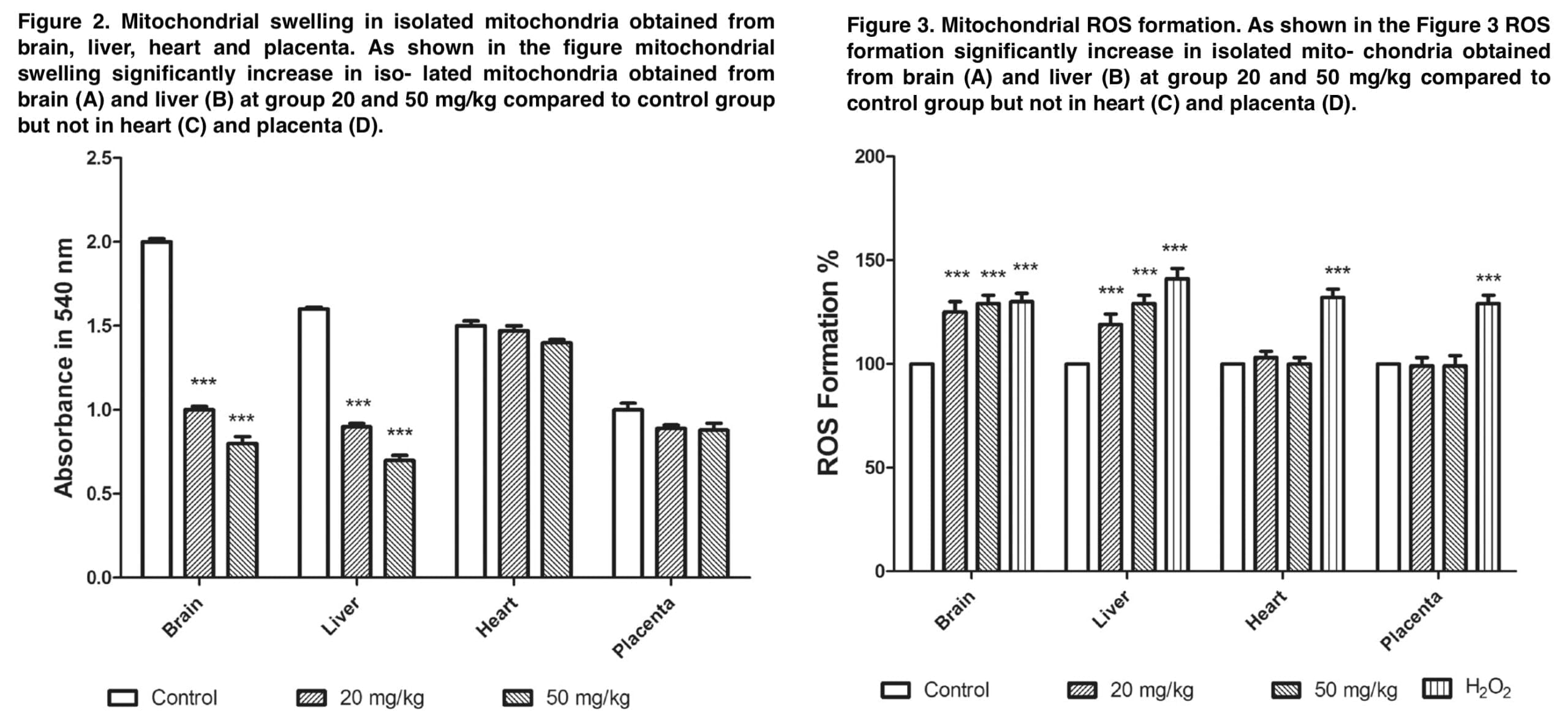
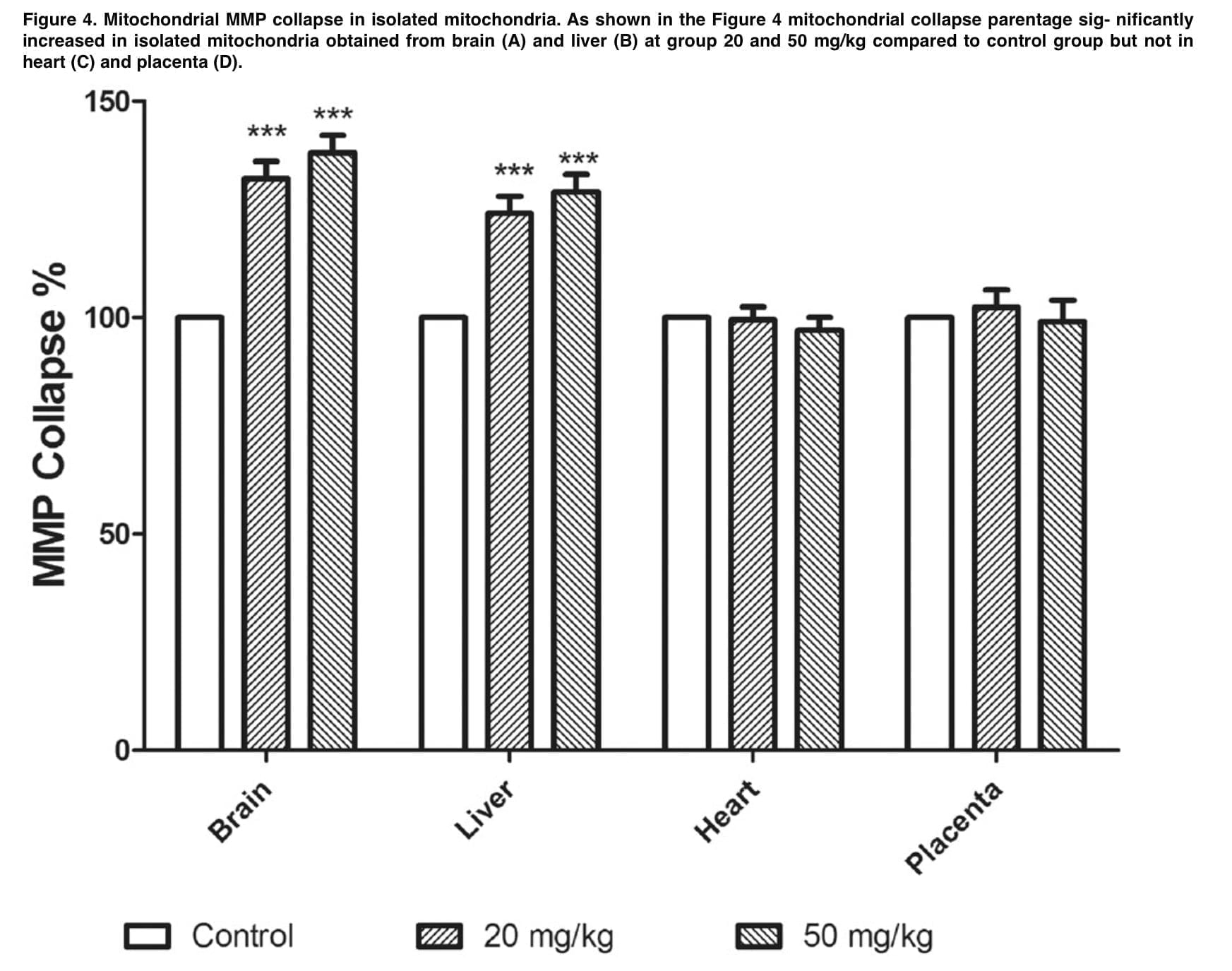
The results demonstrate that mephedrone does cause toxicity and abortion in 20% of cases at a dose of 20 mg/kg. The appendices to this publication (Figure 1 and Table 1) show microphotographs of stained sections with developmental abnormalities in the control and mephedrone-treated groups. Fetuses with macroscopic abnormalities were identified: hepatomegaly, hyperemia, macrocephaly, umbilical hernia, and endometrial thickening. In the groups that received mephedrone, placental weight, placental diameter, and fetal length were statistically significantly reduced relative to the control group. The doses at which there were the above changes in the placenta were 20 and 50 mg/kg. At the same time, there were no such changes at the doses of 1.5 and 10 mg/kg. When assessing the mitochondrial cycle, we found a decrease in uptake at 540 nm, which was an indicator of mitochondrial swelling, which was a criterion of mitochondrial membrane permeability. Doses of mephedrone of 20 mg/kg revealed a significant increase in mitochondrial swelling in isolated suspensions of mouse fetal brain and liver mitochondria compared with the control group, but no such changes were detected in the isolated mitochondria of the heart and placenta.
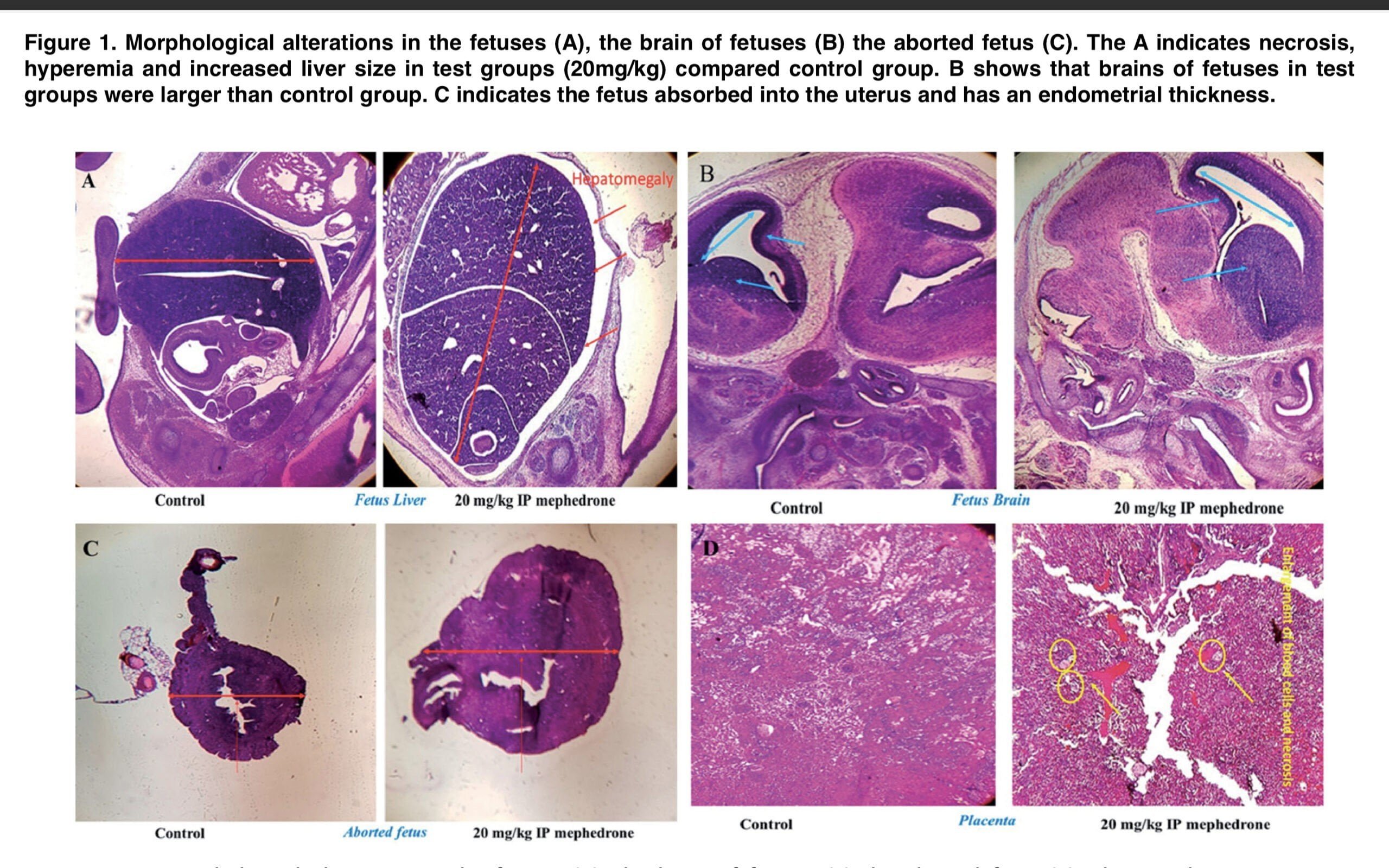
Figure 3 demonstrates that at a dose of 20 mg/kg, mephedrone significantly increases ROS formation in mitochondria that were isolated from mouse fetal brain and liver. In the MMP assay, we measured the degradation of mitochondrial membrane potential by studying the uptake of the cationic fluorescent dye rhodamine 123. Figure 4 demonstrates that mephedrone significantly decreases MMP in mitochondria derived from mouse fetal mogosa and liver compared with controls. Toxic abortion is an important aspect of evaluating the toxicity of various substances. Such a condition is defined as the medical phenomenon of spontaneous abortion or stillbirth caused by toxins during pregnancy. The results of our study clearly demonstrate that mephedrone at a dose of 20 mg/kg causes a toxic abortion. Also, our data showed a statistically significant increase in fetal abnormalities, with the most significant histopathological changes recorded in the fetal brain and liver. In most cases, histological changes included centrilobular hypertrophy and acidophilic cell staining, as well as. the presence of basophilic or clear cell foci.
As we know, mitochondria are important organelles for ATP production, and we have long known about their involvement in programmed cell death. As in prokaryotes, this molecule can be formed in two ways: as a result of substrate phosphorylation in the liquid phase (e.g., during glycolysis) or in the process of membrane phosphorylation associated with the use of energy of the transmembrane electrochemical proton gradient (hydrogen ions). Mitochondria implement both of these pathways, the first of which is characteristic of the initial processes of substrate oxidation and occurs in the matrix, and the second one completes the processes of energy formation and is associated with the mitochondrial crystals. The peculiarity of mitochondria as energy-forming organelles of the eukaryotic cell determines the second pathway of ATP generation, which is called “chemiosmotic coupling. In people suffering from mitochondrial toxicity, cellular dysfunction occurs, leading to both mild and severe problems in the patient. The most common symptom is muscle weakness (myopathy). Other symptoms include peripheral neuropathy (numbness in the fingers and toes) and pancreatitis (inflammation of the pancreas); in the most severe states there is lactoacidosis, in which accumulation of lactic acid in the body tissues leads to loss of energy, disruption of internal organs and ultimately death. Mitochondrial toxicity is a key “off-target” effect of many chemicals and toxicant. These agents have now been identified that inhibit mitochondrial transport pathways, uncouple the electron transport chain (ETC), inhibit fatty acid oxidation or uptake, the citric acid cycle, mtDNA replication, and mitochondrial protein synthesis and generation of ROS. To date, the scientific community has information on only a few factors that influence the vulnerability of mitochondria to toxic substances. One of these factors is the accumulation of a toxicant in mitochondria containing cytochrome P450 and mtDNA. Our histopathological results showed that mephedrone causes damage in the liver, brain, and placenta. Moreover, mephedrone causes significant effects on mitochondrial toxicity parameters (ROS formation, MMP destruction, mitochondrial swelling) in the placenta and mouse fetal brain.
The results of our study unequivocally approved and proved that the dose of 20 mg/kg causes impairment of embryo development in mice. We saw that the liver, brain, and placenta were the main targets most affected by the toxic effects of mephedrone, as evidenced to us by the increased weight and histologically detected cellular changes (centrilobular hypertrophy and so on). Given our findings, we state that mephedrone causes mitochondrial dysfunction in the liver and brain, which causes mephedrone-mediated embryo-fetal toxicity in mice. The above results are useful for awareness and prevention of such toxic effects of mephedrone, especially for pregnant people who use cathinones.