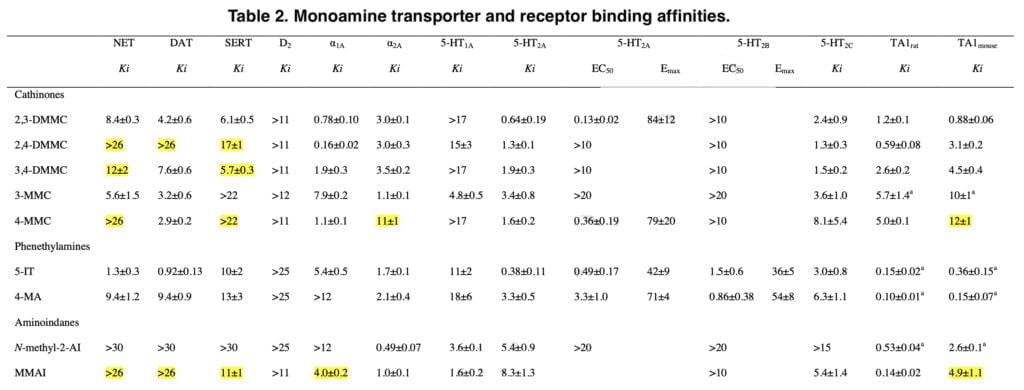
Pharmacological profile of mephedrone analogs
Pharmacological characterization and description of known analogs of mephedrone. A substituted synthetic cathinone (β-keto amphetamine) that has lately gained popularity as a party drug is 4-Methylmethcathinone (4-MMC, mephedrone). Mephedrone was frequently advertised as a “legal high” and remained accessible on the black market even after being declared illegal.
New psychoactive substances (NPS), which are legal substitutes for the recently outlawed mephedrone, have appeared on the drug market. These NPS are structurally and pharmacologically comparable. User reports and clinical intoxication cases are frequently the only sources of information on the effects and toxicity of NPS; pharmacological and toxicological data are mostly absent.
As a result, evaluating the in vitro pharmacological profiles of NPS is a good starting point for learning more about their toxicity and clinical consequences. We compared a novel series of mephedrone analogs and related designer medicines to mephedrone in the current investigation by analyzing the monoamine transporter and receptor interaction characteristics of each (Figure 1). While many of the compounds under examination were initially documented in the 20th century, their widespread availability and recreational usage is a relatively new phenomena.
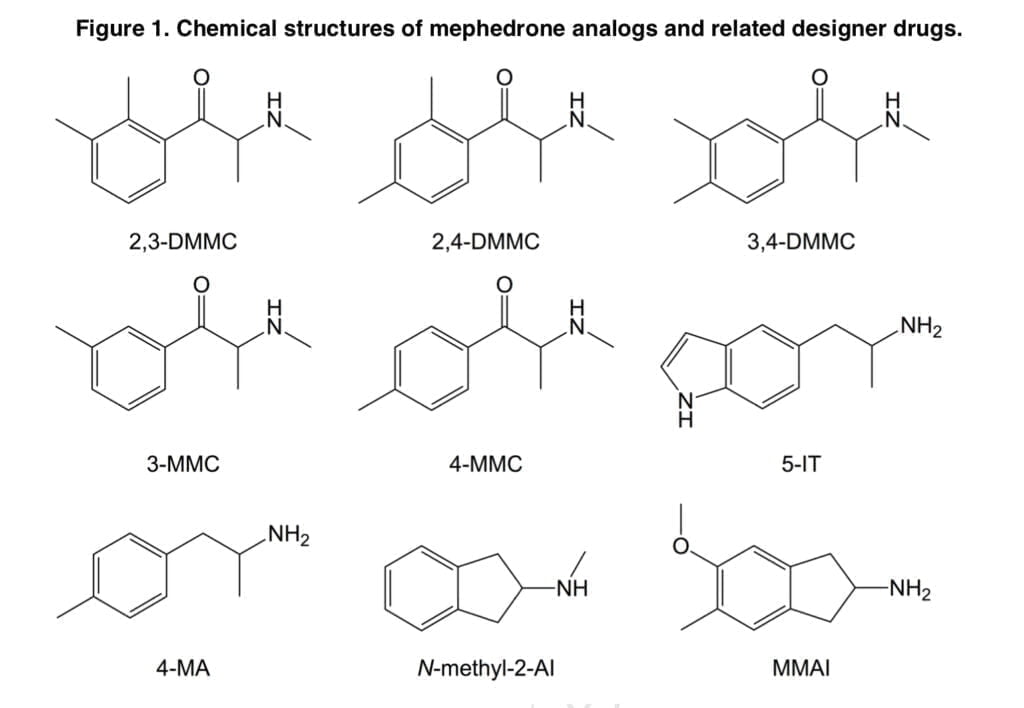
The substituted cathinones 2,3-dimethylmethcathinone (2,3-DMMC), 2,4-dimethylmethcathinone (2,4-DMMC), and 3,4-dimethylmethcathinone (3,4-DMMC) have received relatively little attention to date. Recently, 3,4-DMMC has been marketed and seized in a number of nations. Following the prohibition on mephedrone, 3-Methylmethcathinone (3-MMC) has grown to be one of the most popular NPSs in a number of European nations. It has been linked to clinical toxicity and a number of fatal instances. 5-(2- Aminopropyl)indole (5-IT) is an indole derivative and stimulant NPS that has been associated with numerous fatal and non-fatal intoxications in recent years.
In the synaptosomes of the rat brain, it has been demonstrated that 5-IT is a substrate for the transporters for norepinephrine (NET), dopamine (DAT), and serotonin (SERT), with increased potency for release at NET and DAT over SERT. Additionally, 5-IT gave mice stimulant and locomotor effects comparable to those of 3,4-methylenedioxymethamphetamine (MDMA). The NPS 4-Methylamphetamine (4-MA) has been connected to multiple fatalities when combined with amphetamine and has been found in street amphetamine (“speed”) samples across Europe.
4-MA and d-amphetamine exhibited comparable potencies as releasers of norepinephrine (NE) and dopamine (DA), but 4-MA was a more powerful releaser of serotonin in a research evaluating the monoamine releasing potencies of a series of amphetamine analogs in vitro (5-HT). Rhesus monkeys self-administered 4-MA at a lower rate than d-amphetamine. Two psychoactive aminoindanes have been offered for sale online as designer drugs: N-methyl-2-aminoindane and 5-methoxy-6-methyl-2-aminoindane (MMAI). It has been demonstrated that MMAI has similar effects on the SERT to MDMA and a strong selectivity for 5-HT uptake inhibition vs. NE and DA uptake inhibition.
Materials and methods we used in our study
- Inhibition of the human NE, DA, and 5-HT transporter was assessed in human embryonic kidney (HEK) 293 cells stably transfected with the respective human transporter as previously described. Briefly, cells were cultured in Dulbecco’s modified Eagle’s medium with 10% fetal bovine serum and then resuspended in Krebs-Ringer Bicarbonate Buffer. Monoamine uptake was then quantified by liquid scintillation counting. Nonspecific uptake in the presence of selective inhibitors was subtracted from the total counts.
- Transporter-mediated monoamine efflux was assessed in HEK 293 cells stably expressing the respective transporter as previously described. 100,000 cells per well were cultured overnight in a poly-D-lysine coated cell culture microplate. The cells were then loaded into a special buffer solution containing pargyline, ascorbic acid, glucose, and other compounds, and then processed in the rotor. Monoamine transporter blockers were included in the experiment to determine “pseudo-efflux” caused by nonspecific monoamine release and subsequent reuptake inhibition. The use of a single high concentration and the release durations were based on kinetic evaluation of the release-over-time curves for substrate-releasers in previous studies.
- Cytotoxicity in hSERT-, hDAT-, and hNET-transfected HEK 293 cells was assessed with the ToxiLight bioassay kit according to the manufacturer’s protocol. The cells were treated for 1 h at room temperature with the drugs at the highest assay concentrations. Adenylate kinase release as a result of cell membrane integrity loss was then quantified and compared to control.
- Radioligand binding was analyzed according to Hysek’s methodology. Preparations for HEK 293 cell membranes Ioverexpressing the corresponding transporters or receptors (human genes, with the exception of rat and mouse genes for trace amine-associated receptors) were incubated with radiolabeled selective ligands at concentrations equal to Kd, and ligand displacement by the substances by the incubation was measured. Specific binding of the radioligand to the target was defined as the difference between total binding and nonspecific binding that was measured in the presence of the chosen competitors in excess.
Analysis of the experimental results and discussion
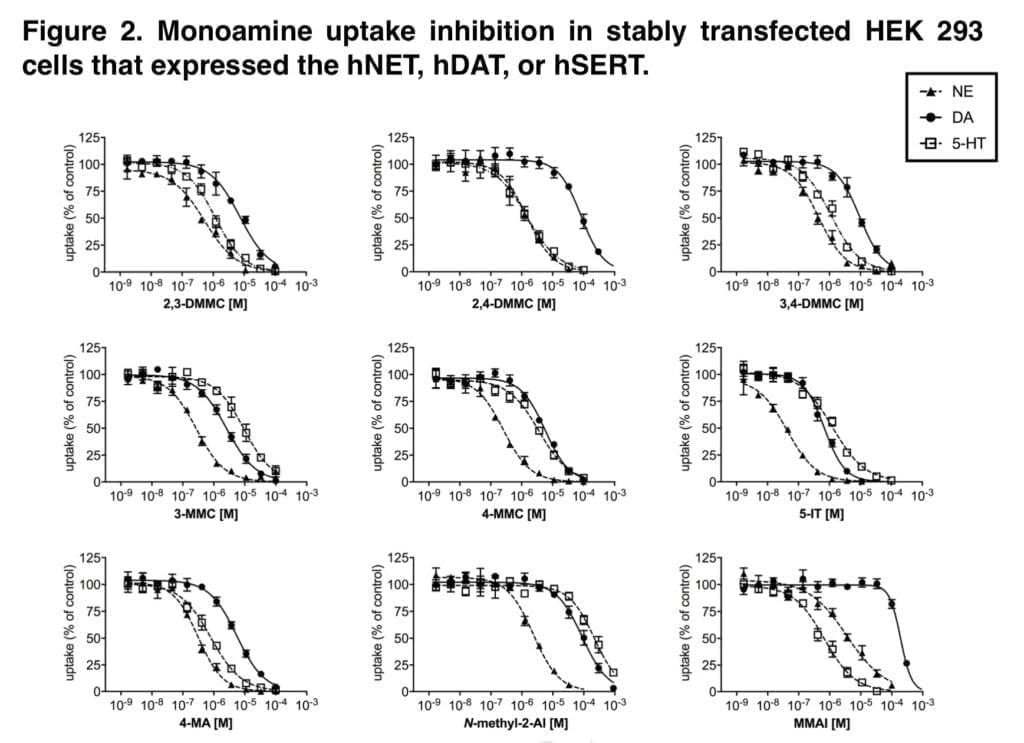
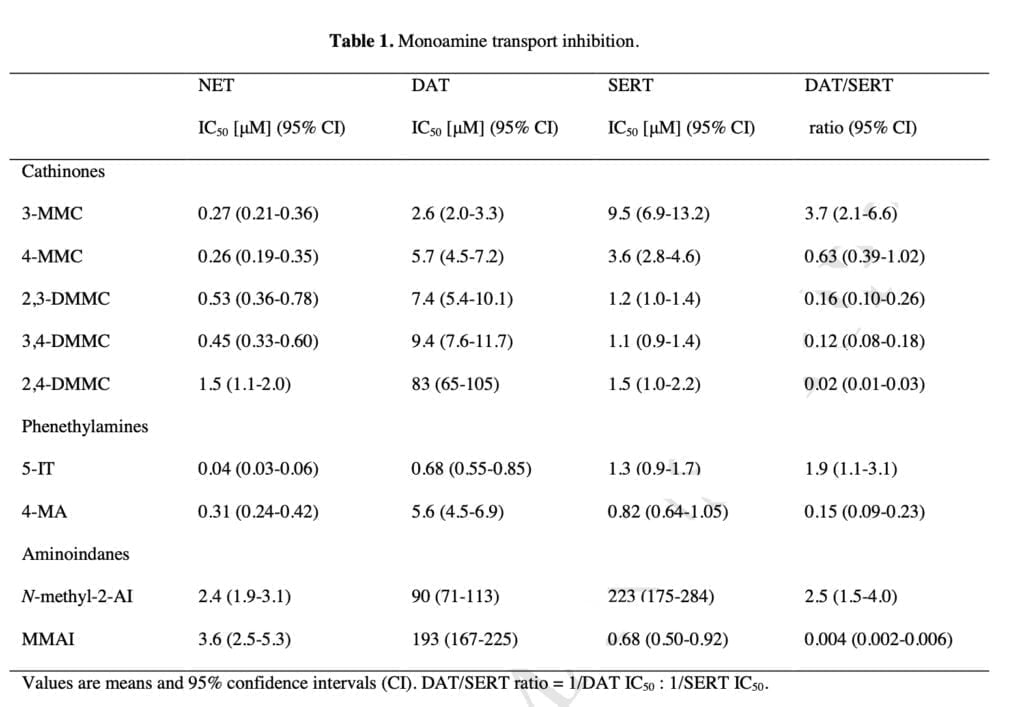
The new mephedrone analogs potently blocked the NET, which is presumably what causes comparable sympathomimetic stimulation as mephedrone does. The discovery that the production of NE but not DA corresponds with human dosages of amphetamine-type stimulants supports the critical role of NE in the acute effects of psychostimulants. Furthermore, in humans, the psychotropic effective dosages of psychostimulants, including cathinones, were closely linked with the NET inhibition potency values.
Furthermore, NE has been shown to contribute to the acute subjective stimulation and cardiovascular effects of MDMA in humans. The DAT was more strongly inhibited by 3-MMC than the SERT. Mephedrone’s efficacy at the DAT and SERT was comparable to that previously seen in some other investigations, while other research revealed that it was 5–10 times more potent at the DAT than the SERT. Additionally, several prior studies did not show mephedrone to have the same strong NET vs. DAT selectivity as it does today.
While our study’s mephedrone selectivity for the NET over the SERT is comparable to previous in vitro studies, the selectivity for the NET over DAT seems to be greater when compared with other laboratories. This has been seen with mephedrone in earlier research from our group, indicating that the variations may be due to variations in the experimental setup or the transfected cell line.
The SERT was more strongly suppressed by the dimethylmethcathinones than the DAT. According to these findings, 3-MMC is more amphetamine-like in its stimulant effects when compared to mephedrone and, in particular, the other, more serotonergic dimethylmethcathinones. Although sometimes mixed with other drugs, stimulant toxicity was observed to be the major clinical hallmark in individuals with recreational 3-MMC overdose. Given that the 5-HT system is more strongly activated in dimethylmethcathinones than in MDMA, these compounds are likely to exhibit entactogenic effects similar to MDMA.
In contrast to earlier research that discovered strong inhibitory selectivity for the DAT vs. SERT for amphetamine, high selectivity for the SERT vs. DAT was also seen for the para-substituted 4-MA. It has been theorized that 4-MA’s significant serotonergic action reduces its reinforcing potency in comparison to other amphetamine analogs. However, consumers of 4-MA tainted “speed” may have experienced some fatal occurrences as a result of the strong serotonergic activity of 4-MA mixed with the significant dopaminergic activity of amphetamine.
Furthermore, the great serotonergic potency of 4-MA, which amphetamine does not share, may help to explain the severe hyperthermia that is seen in these individuals. The NET was effectively inhibited by 5-IT, which also effectively inhibited the DAT and SERT. Serotonergic and sympathomimetic toxicity have both been linked to 5-IT. With relatively moderate inhibitory potencies for the DAT and SERT, N-methyl-2-AI only selectively inhibited the NET, indicating minor psychedelic effects comparable to 2-aminoindane. MMAI had NET inhibition potencies that were similar to N-methyl- 2-AI. Unlike N-methyl-2-AI, however, MMAI potently inhibited the SERT at submicromolar concentrations.
Analogs of mephedrone: substances and scientific results
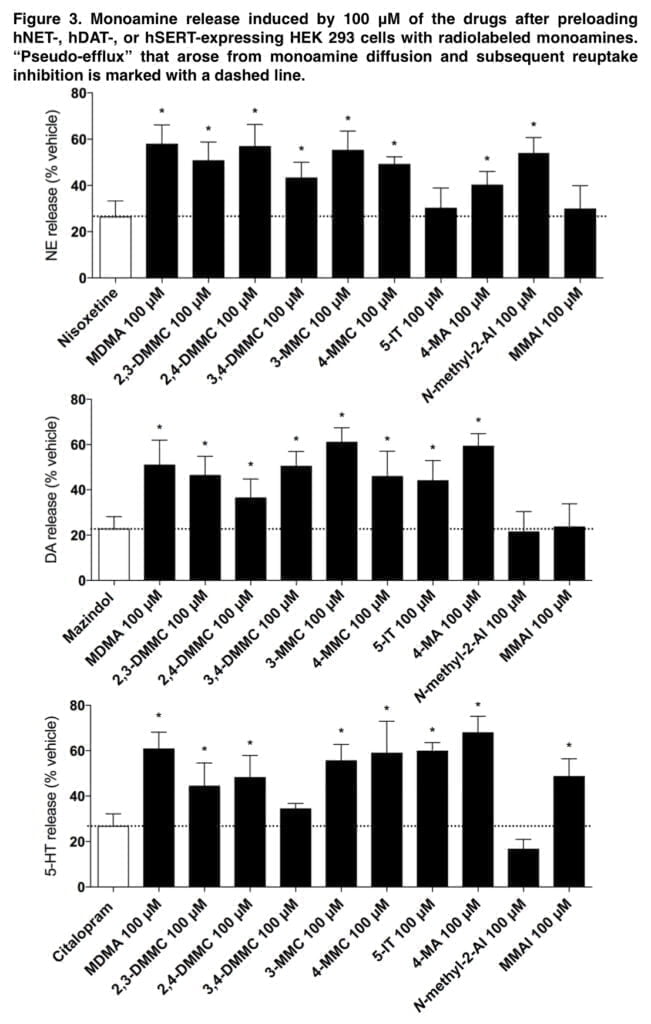
Consistent with previous studies, mephedrone caused efflux of all three monoamines. The cathinone analogs of mephedrone were also monoamine releasers, indicating that they are monoamine transporter substrates like most amphetamines. Although 3,4-DMMC and MDMA have comparable monoamine transporter inhibition profiles, changes in 5-HT release may help to explain some of the discrepancies in their potency and subjective effects. As with amphetamine, 4-MA released all three monoamines.
NE release was not seen, although 5-IT was a highly powerful NET inhibitor. N-methyl-2-AI was a selective NE releaser that blocked the NET alone. A very selective 5-HT releaser, MMAI. The high level of MMAI-induced serotonergic activation supports entactogenic effects. However, the absence of any effects on the DA or NE system suggests that MMAI has a different psychopharmacology than common entactogens like MDMA. Adrenergic receptors, which are known to regulate stimulant-induced behavior, were potently bound by all of the medications.
The medications also affected a number of serotonin receptors. As was previously demonstrated for mephedrone and MDMA and normally for serotonergic hallucinogens, all of the substances bind to the 5-HT2A receptor. Additionally, in our calcium mobilization experiment, 2,3-DMMC, mephedrone, and 5-IT were strong functional 5-HT2A agonists, just like MDMA and other serotonergic hallucinogens that are known to induce their psychotropic effects by at least partially activating 5-HT2A receptors. In a different 5-HT-induced inositol monophosphate production experiment, a different research found mephedrone to have 5-HT2A receptor antagonistic characteristics.
However, MDMA had both agonist and antagonist effects in this assay indicating that the 5-HT2A ligands may act as agonist and antagonists depending on assay set-up. Certain hallucinogenic properties have been described for mephedrone and our results suggest that 2,3-DMMC could have hallucinogen-like properties as well. 5-IT is a positional isomer of the psychedelic tryptamine α-methyltryptamine (αMT). 5-IT has been previously suggested to also have hallucinogenic properties, and its potent 5-HT2A receptor activation supports this possibility. All of the substances interacted with rat and mouse TAARs. Many stimulant NPS interact with TAARs, which have a modulatory role on monoaminergic activity. In a recent screening of a large set of NPS, cathinones were described as poor TAAR1 ligands.
There are restrictions on this study. First off, we didn’t examine how the medications affected intracellular targets like the vesicular monoamine transporter 2. (VMAT2). Methcathinone interactions with VMAT2 have been observed to be less potent than those with MDMA and methamphetamine. Therefore, it was determined that mephedrone is unlikely to result in the release of neurotransmitters from synaptic vesicles.
Second, the static monoamine release test utilized in the current investigation was ineffective for evaluating the potency of the releasers and was only useful for qualitatively determining if a medication is a substrate releaser or not. It would be better to use superfusion assays to gauge the drugs’ ability to release monoamines. However, the potency of the substances to release monoamine is reflected by their potency to inhibit monoamine uptake in the uptake assay used in the present study.
Methcathinone also released monoamines from the DAT more effectively than SERT did in vitro, raised extracellular DA more effectively than 5-HT did in rat brain nucleus accumbens dialysate, and was a more powerful inhibitor of the DAT than SERT. In contrast, the main 5-HT releaser and more powerful in vitro SERT than DAT inhibitor 4-trifluoromethylmethcathinone (4-TFMAP) raised 5-HT but not DA in vivo. As a result, the in vitro profiles for a number of cathinones correctly predicted the in vivo neurochemical effects.
Conclusion
The current work described a number of new mephedrone analogs that had robust interactions with monoamine transporters and receptors, indicating that they may be misused, as has been seen with synthetic cathinones in the past. 4-MA is a strong SERT inhibitor, which may help to explain why it is more hazardous when coupled with amphetamine, a strong DAT inhibitor. Highly effective monoamine transporter inhibitor 5-IT has been linked to sympathomimetic toxicity and a large number of fatalities throughout Europe. Both NE and SERT are selectively inhibited by N-methyl-2-AI, whereas SERT is selectively inhibited by MMAI and 5-HT is released.
