Mephedrone metabolism (Mephedrone Bioavailability) has been the subject of in vitro and in vivo research in both animal models and humans. N-demethylation of the secondary amine results in nor-mephedrone (NOR), reduction of the ketone moiety to the hydroxyl group results in dihydro-mephedrone (DHM), and oxidation of the tolyl moiety results in hydroxytolyl-mephedrone (HYDROXY) and 4-carboxy-mephedrone (4-CARBOXY). Dihydronormephedrone is created when the ketone moiety is simultaneously reduced, and the secondary amine is N-demethylated (DHNM).
Human mephedrone metabolism was discovered to be primarily mediated by the hepatic CYP2D6 enzyme, with minimal involvement from other CYP enzymes. Mephedrone’s stability has previously been studied in human whole blood that has been preserved with various agents and kept in various settings. When mephedrone is stored using acidic preservatives (NaF/KOx and NaF/citrate buffer), it has been shown to be best stable at -20°C.
Although the exact reason for mephedrone’s instability in biological matrices is unknown, a prior investigation on the drug’s breakdown in alkaline solution points to the participation of oxidants such dissolved oxygen.
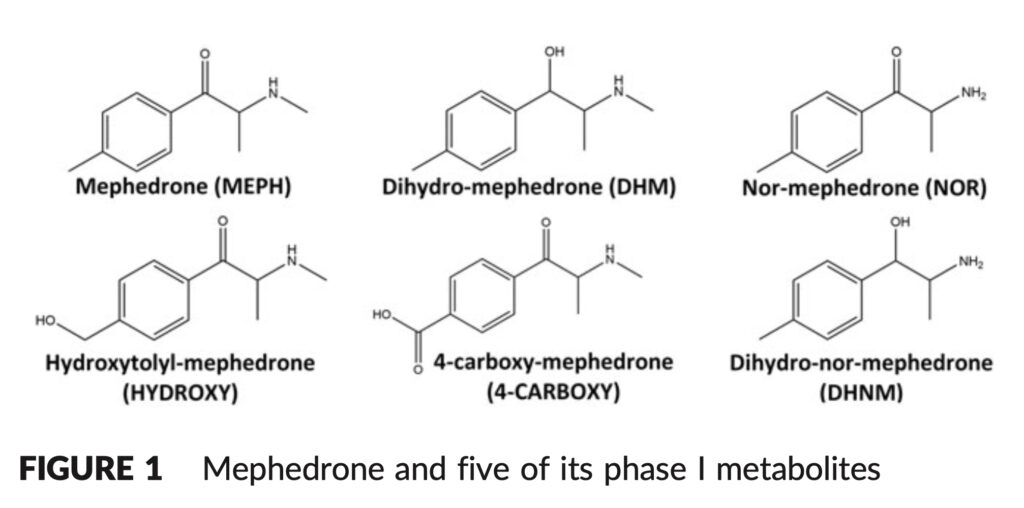
The stability of mephedrone and five of its phase I metabolites were examined in this work in human whole blood that had been supplemented with NaF/KOx as a preservative and anticoagulant, respectively. Since the principal mephedrone metabolites in whole blood have not before been the subject of a comprehensive stability study, this is a significant work that will be useful to both clinical and forensic toxicologists.
Mephedrone Bioavailability: Research Methods
In the present study, we used the following substances: Mephedrone hydrochloride (MEPH), dihydro-mephedrone hydrochlo-ride (DHM), mephedrone-d3 hydrochloride (MEPH-d3), dihydro-mephedrone-d3 hydrochloride (DHM-d3), 4-(2-aminoethyl) benzoic acid hydrochloride (AEBA) and sodium borohydride. Ten mg of NOR (61.3 μmol) was reduced to DHNM. Synthesized product was stored at −40°C and a small amount was characterized using high resolution mass spectrometry (HRMS) to determine its accurate mass. One hundred μL of whole blood (NaF/KOx) was extracted using solid‐ phase extraction (SPE).
Ten μL of IS or 10 μL of MeOH:water (50:50 v/v) was added to the samples and solvent/matrix blanks, respectively. All samples were vortex mixed and 1 mL of 0.1 M phosphate buffer (pH 6.0) was added. After conditioning the SPE cartridges (mixed mode cation exchange containing C8 and benzoylsulfonate anion) with 2 mL of MeOH and 2 mL of 0.1 M phosphate buffer (pH 6.0), samples were loaded and washed with 2 mL of 0.1 M acetic acid(aq) followed by 2 mL of MeOH.
Stability samples made in drug-free human whole blood (NaF/KOx) at QC Low and QC High levels were aliquoted into tubes and kept at +4°C and -20°C for 24 hours, 48 hours, four days, and ten days. Six aliquots were collected from one tube at each QC level at each sample point. Temperatures in the refrigerator and freezer were tracked daily.
Matrix‐matched calibration curve was prepared by fortifying drug‐free whole blood (NaF/KOx) with appropriate working solutions containing mephedrone and its metabolites. The correlation coefficient (r2) of the line had to be at least 0.99, and each calibration standard had to be within 20% of its target concentration. On the calibration curve, a linear regression model was used with a weighting of 1/x.
A set of blank whole blood samples (n = 6, from three female and three male participants) and a set of water samples (n = 6) were collected through the extraction for the IS-corrected matrix effect. A solution containing known concentrations of the internal standard and the analytes at QC Low and QC High levels was used to reconstitute all samples. By contrasting peak area ratios in water samples fortified after extraction with peak area ratios in blank whole blood samples fortified after extraction, the matrix impact was assessed.
Discussion of the results
DHNM was successfully synthesized (yield: 51%). Formula C10H16NO+; HRMS [M + H+] calculated m/z 166.1226, observed 166.1227 (+0.001 ppm); observed MS/MS fragments with collision energy 20 eV were consistent with those reported in the literature. 1H NMR (CDCl3): δ 7.22 (d, J = 8.0 Hz, 2H, Ar‐H), 7.16 (d, J = 8.0 Hz, 2H, Ar‐H), 4.50 (d, J = 4.0 Hz, 1H, CH (OH)), 3.19 (br, 1H, CH (CH3)), 2.35 (s, 3H, Ar‐CH3) and 0.98 (d, J = 8.0 Hz, 3H, CH (CH3)).
NMR data is consistent with the literature except for the signal at 3.19 ppm being previously reported as a multiplet. No interferences were observed in the extracted blank matrix. Mean linearity of r2 > 0.998 was achieved for each analyte in all three validation runs. LOD and LOQ of 50 pg/mL and 200 pg/mL, respectively, was achieved for all analytes except 4‐CARBOXY for which the LOD was 500 pg/mL and the LOQ 2000 pg/mL. The discrepancy in order of magnitude found was most likely caused by the amphiprotic molecule’s lower electrospray ionization efficiency under the selected mobile phase conditions. For each analyte, calibration parameters are shown in Table 2.
Results for intraday and interday precision and accuracy, compiled in Table 4, were discovered to be within acceptable bounds. The intra‐day accuracy for all metabolites was within ± 15% of the target concentration and ranged from 96.7% to 106% for MEPH, 91.1%–109% for DHM, 89.7%–97.0% for NOR, 94.3%–115% for HYDROXY, 97.0%– 114% for 4‐CARBOXY, and 86.6%–103% for DHNM. The intra‐day precision was <7% and ranged from 1.44% to 4.33% for MEPH, 0.924%–4.65% for DHM, 1.58%–4.87% for NOR, 1.55%–6.57% for HYDROXY, 1.36%–5.97% for 4‐CARBOXY, and 1.52%–5.13% for DHNM. With % RSD 8.5% and accuracy within 9.0% of the target concentration, inter-day precision and accuracy values were acceptable across the validated range.

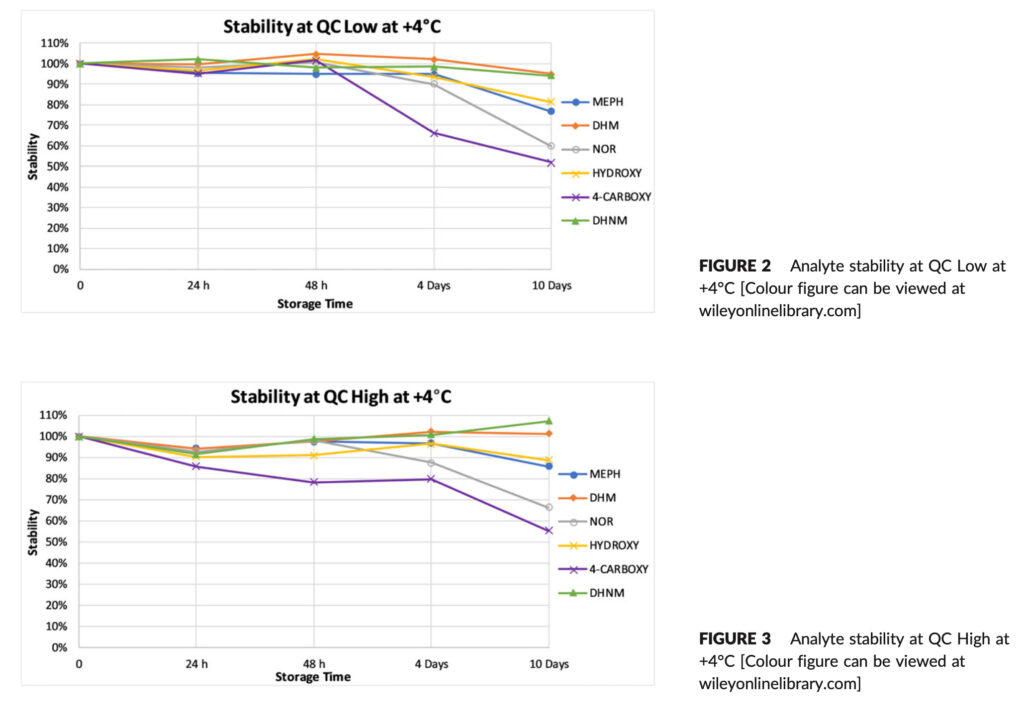
Figures 2–5 display the stability data visually and in Table 4 the corresponding percent RSD. When an analyte dropped more than 10% of its initial concentration, it was deemed unstable. DHM and DHNM were constant at +4°C at QC Low over a period of 10 days, however HYDROXY and MEPH lost 18.6 and 23.4%, respectively, of their starting concentration.
4‐CARBOXY and NOR decreased in concentration by 48.1% and 40.2%, respectively, after 10 days (Figure 2). At QC High, DHM and DHNM were stable over the 10‐day period while HYDROXY and MEPH lost 11.3% and 14.2%, respectively, of their initial concentration. 4‐CARBOXY and NOR were most unstable and their concentration decreased by 44.6% and 33.8%, respectively, after 10 days (Figure 3).
The most unstable metabolite overall at +4°C was 4CARBOXY, with considerable losses seen as early as four days (33.7%) at QC Low and 48 hours (21.6%) at QC High. At 20°C, where the maximum loss of 22.6% was seen after 10 days at QC High, its stability was enhanced, NOR was significantly more stable at 20°C than it was at 4°C, where it lost 40.2% at QC Low after 10 days and 33.8% at QC High after 10 days.

After 10 days, HYDROXY was unstable in both storage environments, with QC Low at +4°C seeing the maximum loss of 18.6%. DHM and DHNM were stable at both concentration levels when refrigerated, with the latter displaying a modest rise in its concentration after 10 days at QC High, in contrast to other analytes which exhibited considerable losses at +4°C after 10 days.
Ephedrines are known to be more stable than cathinones, and DHM and DHNM are the only two metabolites that have a hydroxyl group rather than a ketone at the carbon. Mephedrone stability studies in human whole blood have been published in the past. Samples were kept for up to five or six days at +4°C or +20°C. Mephedrone was more stable at +4°C than +20°C after five days of storage. In ante-mortem samples, mephedrone was shown to be most stable under all studied storage settings, with -20°C being the ideal storage temperature.
According to this study, the stability of mephedrone is pH-dependent, and acidic preservatives are preferable (6.6% vs. 9.4% loss after 185 days at -20°C when preserved with NaF/KOx rather than EDTA). Human whole blood, plasma, and urine samples were spiked at 1 g/mL with an unspecified preservative. According to the study, mephedrone levels in whole blood stored at +4°C for 14 days decreased on average by 48%.
In a recent research, mephedrone and other synthetic cathinones were tested for stability in bovine blood that had been fortified with NaF/KOx at concentrations of 100 ng/mL (QC Low) and 1000 ng/mL (QC High) and held at -20°C, +4°C, +20°C, and +32°C. Mephedrone was found to completely degrade at QC Low after 11 days of storage at the high temperature. At +4°C and -20°C, where degradation was significantly slower, a 20% loss was found after 55 days and 130 days, respectively. These findings support the stability pattern found in our investigation, which demonstrated that mephedrone and its metabolites are more stable at a temperature of -20°C than +4°C.
Summary
The QCs used for the stability study were the same as those used in the pharmacokinetic experiment, which examined the entire blood of five healthy volunteers who had inhaled a dosage of 100 mg of mephedrone powder. Although the findings from that study are now being processed for publication, it is pertinent to note that the individuals’ whole blood metabolite concentrations were comparable to the amounts selected for the low, medium, and high quality controls.
To produce values within the calibration range and around the mephedrone concentrations in the QCs, as well as to prevent ion overload, sample dilution was predicted and in fact necessary for the assessment of mephedrone concentrations in the pharmacokinetic research. Our stability study concentrated on mephedrone metabolites, and to the best of our knowledge, the findings are brand-new. However, while being at considerably lower concentrations than those generally associated with acute toxicity, our results regarding the stability of mephedrone seem to be consistent with earlier stability studies that used larger doses.
