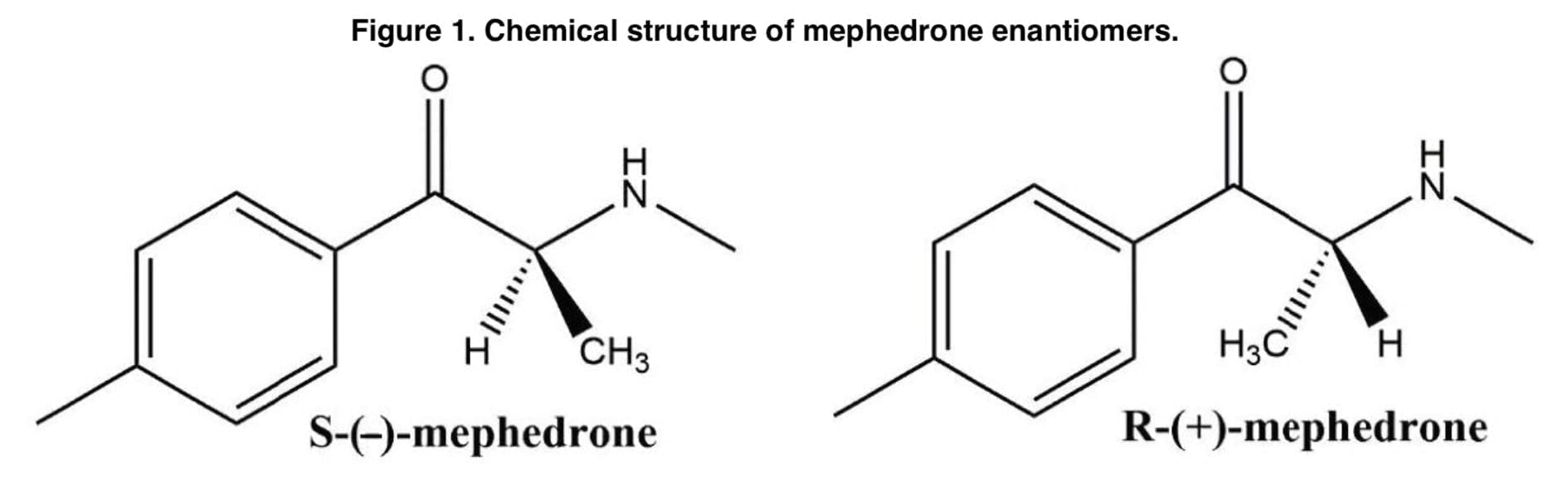
In the preface to this publication, we want to emphasize Figure 1, which demonstrates a mephedrone existing in two enantiomers and having one chiral center in α-carbon: R-(+)-mephedrone (R-MEPH) and S-(-)-mephedrone (S-MEPH). The basic synthetic process of mephedrone involves the alpha-bromination of 4-methylpropiophenone followed by a reaction with methylamine hydrochloride and triethylamine. Next, there is treatment with hydrochloric acid gas followed by recrystallization to obtain the hydrochloride form. This method produces a racemic mixture but a stereoselective synthesis via Friedel–Crafts acylation can also be performed.
Since it is easiest to synthesize a racemic mixture of mephedrone, this is the form in which it is sold on the street. In most cases, enantiomers differ in their pharmacodynamic activity. For example, one enantiomer may be biologically active, while another may be inactive and, sometimes, may even cause negative and undesirable effects. Although preliminary data from in vitro studies, animal studies, and analysis of human urine suggests that mephedrone enantiomers may have a different metabolic pathway and exhibit different neurological effects, to our knowledge there are no published reports on the pharmacokinetics of mephedrone enantiomers in humans. According to a study done on rats, R-MEPH exhibits more stimulant-like effects than S-MEPH does because it is more selective for the dopaminergic system. Additionally, mephedrone’s phase 1 metabolites have bioactive qualities, with the stereoisomers blocking the uptake of dopamine, norepinephrine, and serotonin through transport-mediated absorption. It is noteworthy that studies have shown that nor-mephedrone and 4-hydroxytolyl-S-enantiomers mephedrone’s are far more powerful inhibitors of serotonin-mediated absorption than their R-enantiomers. Overall, determining the degree of asymmetry in the abundance of mephedrone’s enantiomers and their metabolites in biological samples should help shed light on the drug’s pharmacodynamics.
Mephedrone was found in human whole blood and plasma for 6 hours after administration in a study that recently came out, and it exhibited quick absorption and excretion. The controlled intranasal injection of 100 mg of pure racemic mephedrone hydrochloride to healthy human volunteers resulted in the first comprehensive analysis of the pharmacokinetic characteristics of mephedrone enantiomers ever conducted.
Methods of experiment implementation and technical materials used
In our study, we used racemic mephedrone hydrochloride, racemic mephedrone-d3 hydrochloride (MEPH- d3), S-(-)-methcathinone and R-(+)-methcathinone. The chemical structure and purity of the psychoactive substances were confirmed by mass spectrometry and nuclear magnetic resonance. The participants included in the study were users of mephedrone or other psychostimulants; however, they had been drug-free for one week prior to mephedrone ingestion, which was confirmed by appropriate testing. Six healthy male volunteers intranasally ingested 100 mg of mephedrone hydrochloride, a racemic mixture of 96.3% purity. Venous blood was collected in 5 ml volume with preservative for the following time steps: 5 min, 10 min, 15 min, 20 min, 30 min, 45 min, 60 min, 75 min, 90 min, 105 min, 2 h, 2.5 h, 3 h, 5 h, 6 h, Day 2 and Day 3. Sample analysis was performed by liquid chromatography–tandem mass spectrometry using a Xevo TQ-S triple quadrupole mass spectrometer coupled to an Acquity ultra performance liquid chromatograph system.
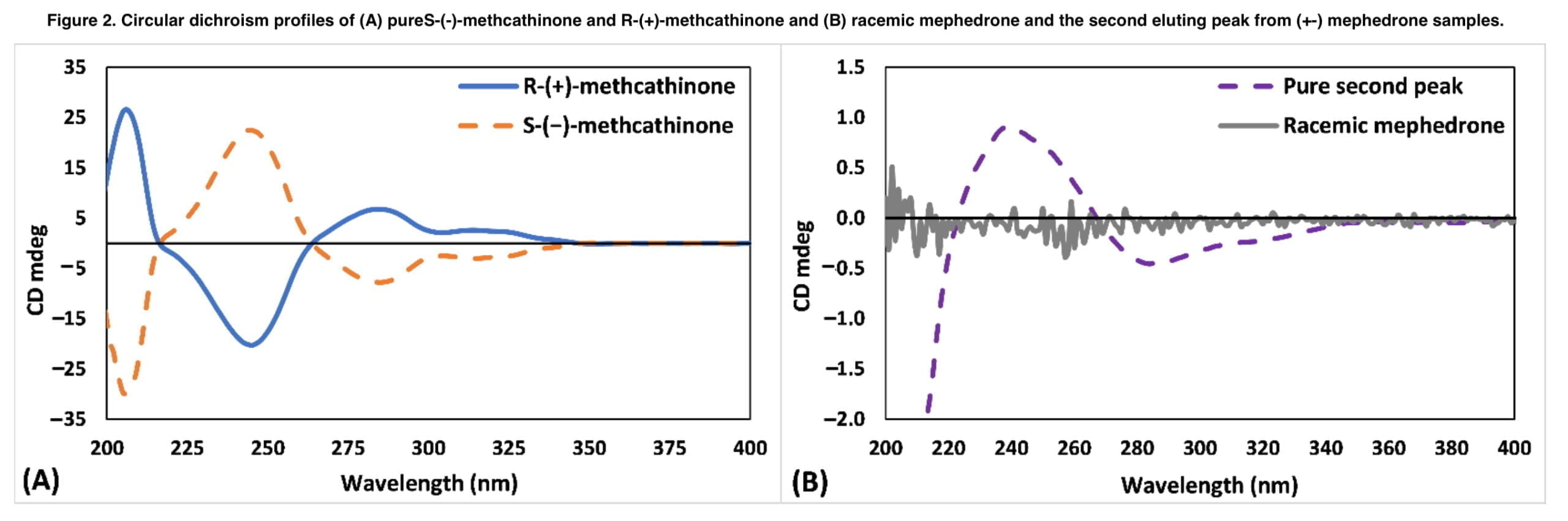
Fractions resulting from a semi-prep chiral chromatography injection were gathered and subjected to CD analysis to ascertain the enantiomers’ order of elution. Because it was not commercially feasible to get pure mephedrone enantiomers at the time of this study, the chemical analogs R-methcathinone and S-methcathinone were analyzed by CD along with mephedrone fractions. The absence of the 4-methyl group on the benzene ring distinguishes methcathinone from mephedrone (Figure 2). The CD spectra of mephedrone fractions were compared with the reference CD spectra of methcathinone enantiomers, which have established an absolute configuration, because the remaining structure, including the chemical environment surrounding the chiral centre, is identical. Validation experiments determined selectivity, linearity, inter- and intra-day precision and accuracy, limit of detection (LOD), lowest limit of quantification (LLOQ), recovery, matrix effect and carryover.
Discussing the results and summarizing the results
As indicated in the tables and accompanying figures, the concentration of the right-hand enantiomer of mephedrone in whole blood had higher Cmax, AUC, and T1/2 parameters, relative to the S-enantiomer. Based on the results of past in vitro and in vivo studies, we can conclude that it is the R-form that leads to more stimulating effects because of its greater selectivity for the dopaminergic system (tentatively, activity is 50 times greater than that of the S-enantiomer). The higher intracranial self-stimulation caused by R-MEPH was also discovered to have stronger abuse-related consequences. As a result of the precursors’ low cost and wide availability, it is believed that “street mephedrone” is sold as a racemic combination. An alternate synthesis pathway (perhaps enantiomerically specific) could be used in the event that the starting material becomes scarce, expensive, or the subject of stringent international regulation. Users of mephedrone may be exposed to higher levels of toxicity if the resulting synthesized product has an enantiomeric excess of R-MEPH.
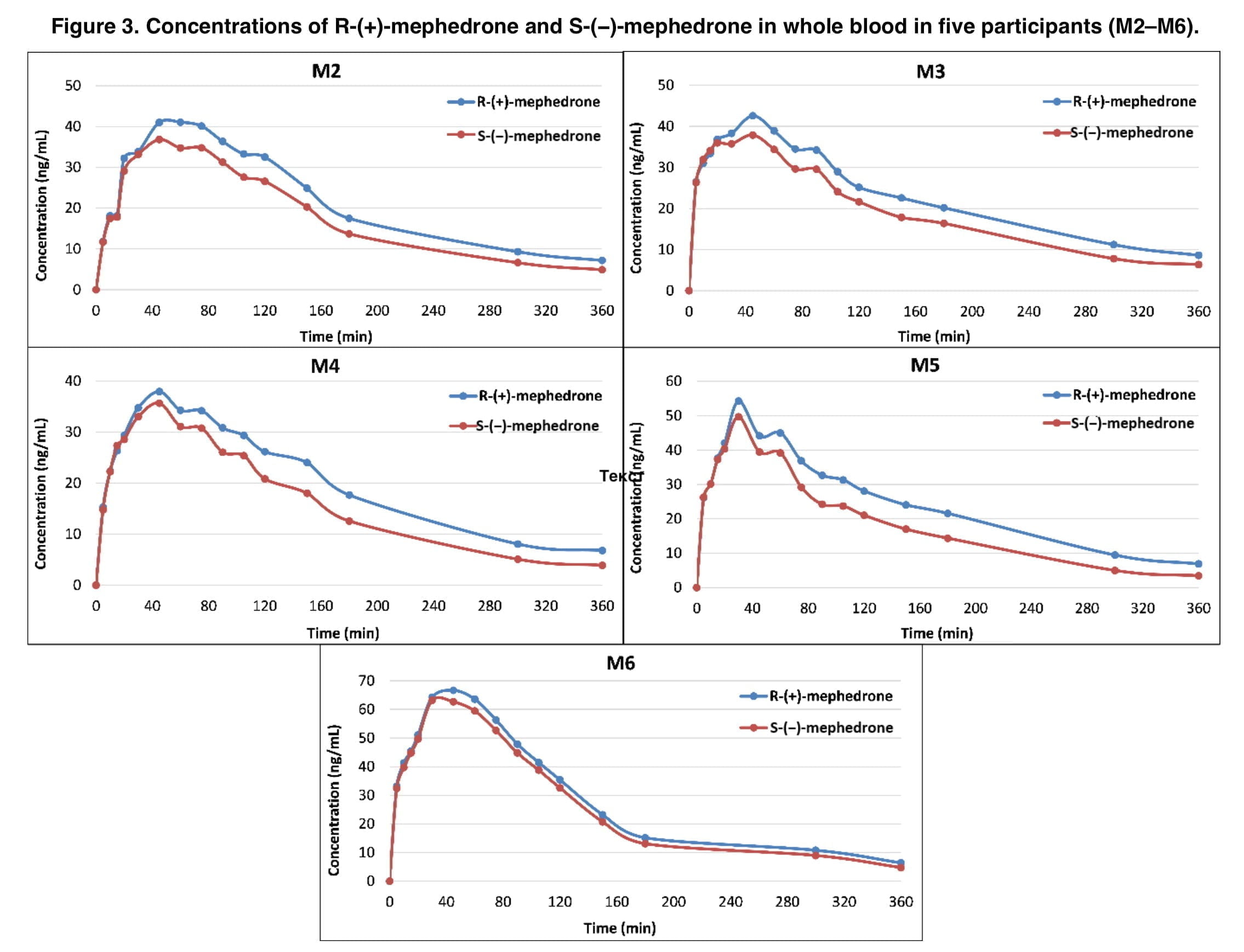
In whole blood, mean racemic mephedrone concentrations were recently shown to be around twice as high as mean concentrations of each enantiomer. When measured in whole blood, R-pharmacokinetic MEPH’s characteristics were more similar to those of racemic mephedrone. In our experiment, both enantiomers peaked after 50 minutes, had the same kel parameter of 0.006 and almost the same T1/2, which was 2.12 hours for the racemic mephedrone and 1.92 for the R-enantiomer. The maximum concentration of racemic mephedrone was 101 ng/mL, twice that of the left-hand form of mephedrone.
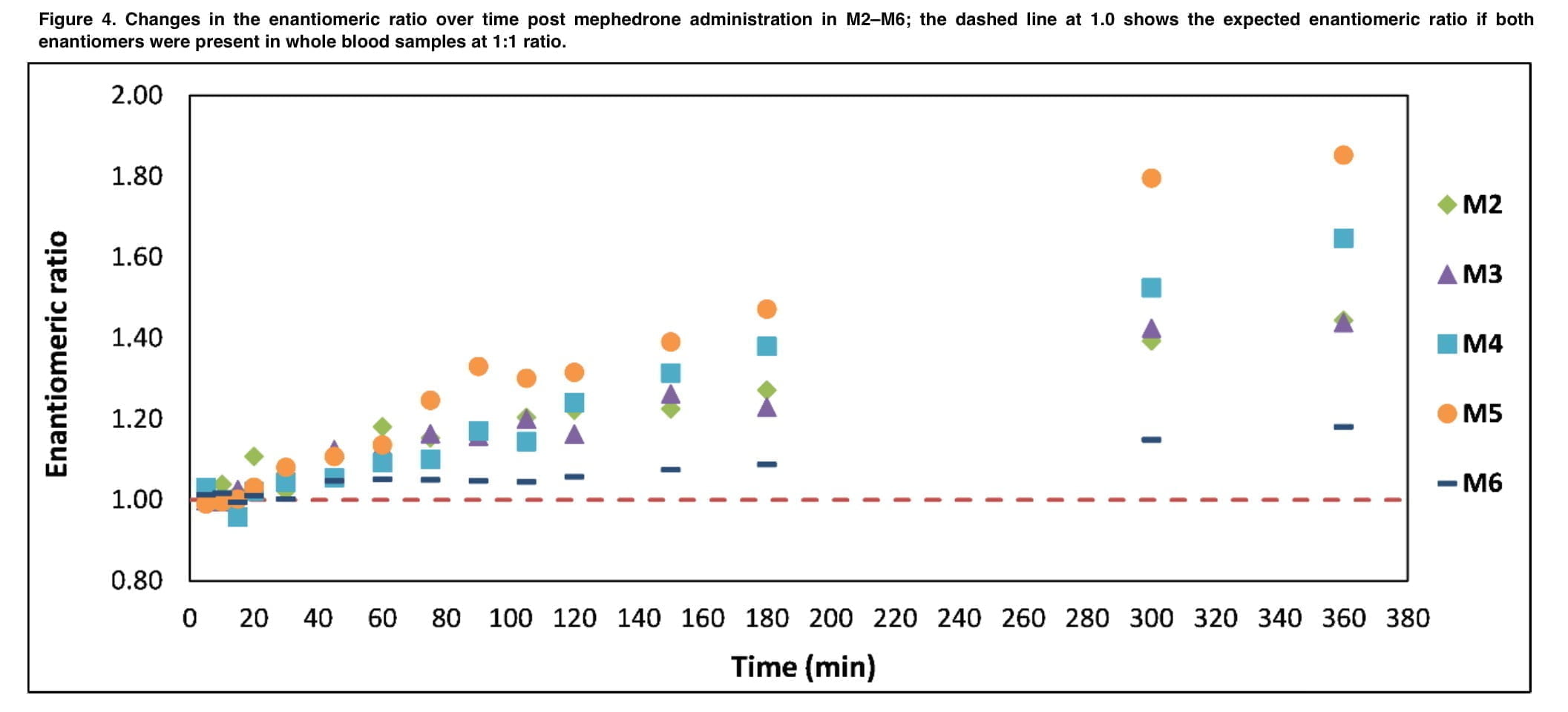
Whole blood samples taken between 45 and 360 minutes later had an ER that was statistically distinct from 1.0, according to changes in the ER with time. Pharmacokinetic activities that take place during medication absorption, distribution, metabolism, or excretion may occur at varying rates, which can lead to variations in the amounts of S-MEPH and R-MEPH. In vivo, certain medications can undergo unidirectional or bidirectional chemical or biological inversions. The concentrations of enantiomers in whole blood may also be influenced by polymorphic drug metabolism, gender, age, illness condition, and drugs, though given the methodology of this study, polymorphic drug metabolism is probably going to have the biggest impact. Genetic polymorphism affects hepatic cytochrome P450 2D6 (CYP2D6), which is in charge of metabolism of mephedrone and results in differences in CYP2D6 enzymatic activity. Unfortunately, the volunteers in our study were not genotyped for the above cytochrome form polymorphism. Despite this, we demonstrated that significantly altered plasma concentrations of mephedrone may be the result of CYP2D6 activity. In addition, those volunteers with low or absent functionality of this enzyme may be at greater risk of acute toxicity.
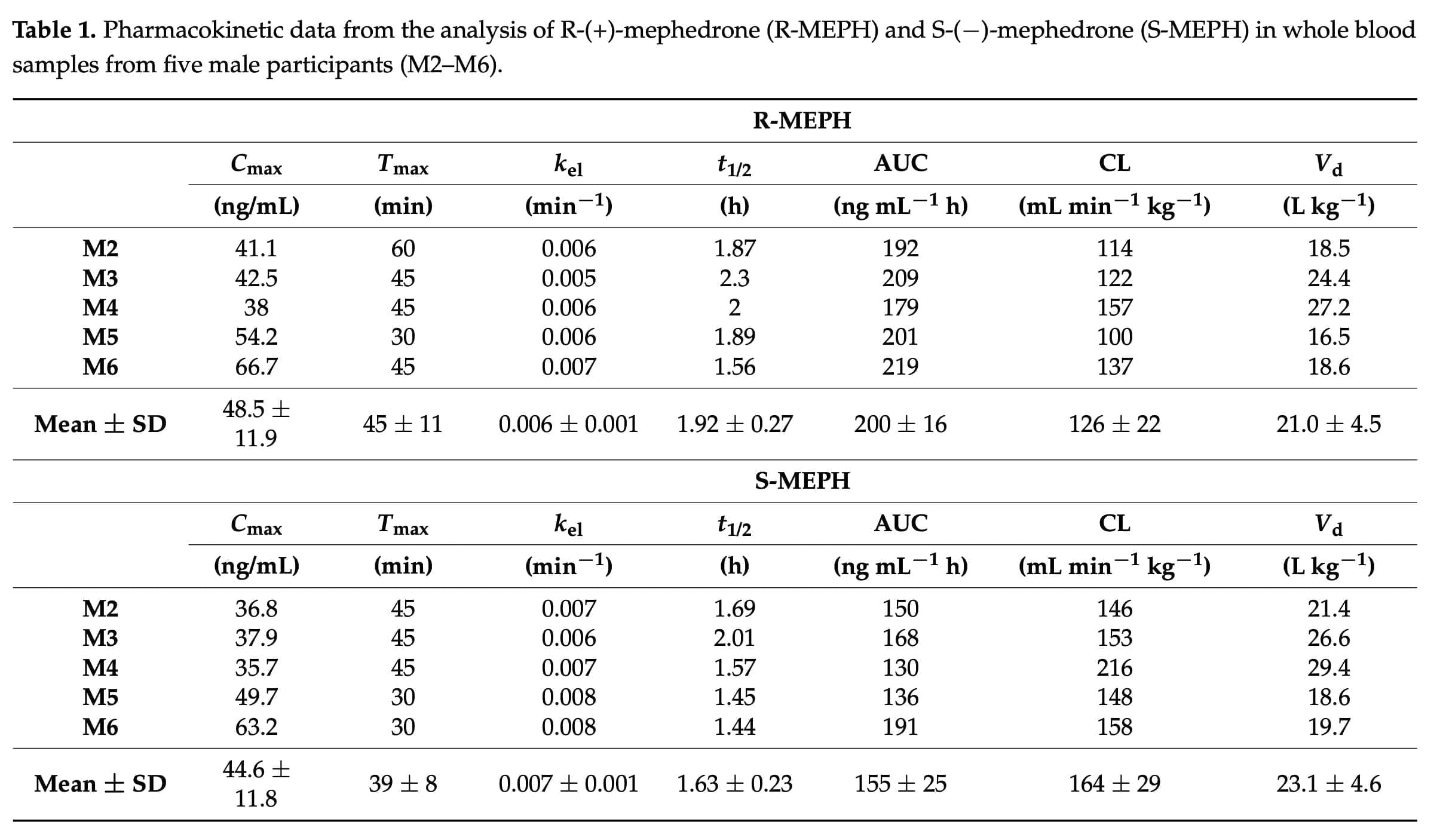
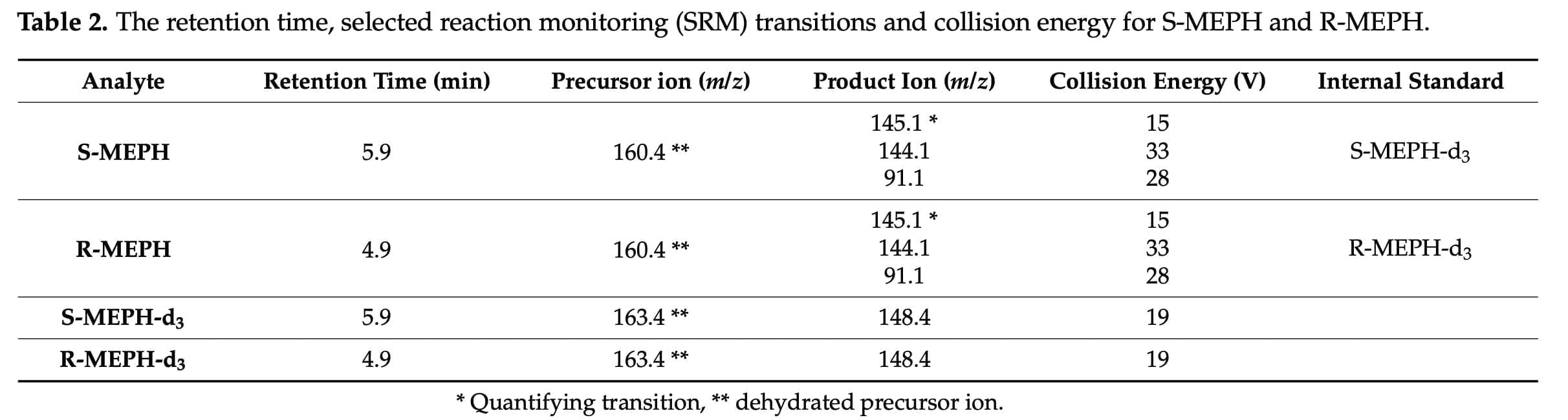
Chiral analysis is still not commonly used as part of standard operating procedures in clinical and forensic toxicology, despite the fact that it is thought to be crucial and occasionally required for appropriate interpretation of analytical findings. In this situation, it could be worthwhile to perform chiral analysis as a follow-up to regular examination of blood samples when mephedrone has been found. To gauge the value of this extra information, particularly in relation to R-MEPH, a few case studies may be conducted at first. Our study demonstrates that mephedrone demonstrated clear enantioselective pharmacokinetics, with the R-form achieving higher maximum concentration, AUC, and T1/2.
