Along with other compounds in the class of psychostimulants, mephedrone bears a striking resemblance in structure and acute action mechanism to methamphetamine. However, it differs from it in structure by the presence of two substituents: a β-keto group and a 4-methyl substitution on the phenyl ring. Also, there are two possible intermediates between them: 4-methylmethamphetamine and methcathinone; these compounds were used in Anneken research to evaluate the neurotoxicity of each of the above substituents. Mephedrone and methamphetamine elicit acute release of dopamine through binding and reversal of the dopamine transporter. This excessive release of DA is thought to contribute to the generation of oxidative species that mediate the long-term toxicity of METH, characterized in rodents as a reduction of markers of striatal dopaminergic synaptic integrity including DA, DAT, and tyrosine hydroxylase. Despite a similar acute neurochemical and behavioral profile, mephedrone has generally been found to lack this lasting striatal toxicity elicited by METH. At this point, the reason for the lack of neurotoxicity of mephedrone remains unclear. In Anneken’s research on the role of endogenous DA, it was found that dopamine is released in very large quantities via the special transporter VMAT2 by methamphetamine than by mephedrone. We anticipated that pharmacologically boosting the pool of releasable DA would cause MEPH to become toxic in a manner akin to METH. Following these tests, MEPH did not exhibit any toxic effects on DA nerve endings, proving that the toxicities of the medications could not be solely attributed to the quantity of DA produced by each substance.
Another effect that defines the criterion for the difference between mephedrone and methamphetamine is the effect on body temperature. It has been established that METH causes hyperthermia, which increases the drug’s long-term toxicity, and that reducing this impact can have neuroprotective effects. Both substances cause hyperthermia when used, but the difference is that it is the mephedrone that causes short-term but significant hypothermia at the outset. Thus, since hypothermia can be a protective factor against toxicity, it may be the key factor protecting against neurotoxicity during mephedrone use.
Shortall’s research found that pharmacologically-induced depletion of serotonin and blockade of its receptors (5-HT1A) attenuates the hypothermic response induced by mephedrone ingestion. That study, however, did not examine how this modification might affect dopaminergic toxicity. Our laboratory has worked extensively with a line of mice genetically depleted of 5-HT through deletion of the gene for tryptophan hydroxylase-2 (TPH2), the initial and rate-limiting enzyme in the biosynthesis of brain 5-HT, so this presented an intriguing and selective way to evaluate the effect of intrinsic hypothermia on the toxicity of MEPH, as well as the related compounds MeCa and 4-MM. In this publication we will tell you about a study that assessed changes in body temperature and DA nerve endings damage in response to administration of mephedrone, MeCa and 4-mm.
Methods of the study
When performing the study, we used Tph2 knockout mice on pure background with confirmation by genomic screening. The mice ranged in age from 8 to 14 weeks, 5 to 7 pieces weighing 15 to 25 g in each cage. We used racemic 4-MM synthesized from methylamine HCL and 4-methylphenylacetone as the main substance; its structure was confirmed by nuclear magnetic resonance and mass spectrometry. Animals were injected intravenously at 2-hour intervals with one of the following substances: mephedrone at a dose of 40 mg/kg; 4-mm at the same dose; MeCa at a dose of 80 mg/kg, and the control substance was saline. The animals are removed from the experiment 48 hours after the last injection. Animal temperatures were studied using telemetry, by means of implantable temperature transponders injected subcutaneously. Body temperature was recorded 24 hours before the start of the experiment and thereafter, according to the scheme. Mice were considered hypo- or hyperthermic if their body temperature differed statistically significantly from the control group.
To identify the dopamine content in the striatum, we separated this part of the brain, froze on dry ice until processed. Then, after centrifugation, we injected 50 μl onto a c-18 reverse phase column in buffer and quantified the peaks according to standards. Levels of DAT, TH, and SERT were determined via immunoblotting. Frozen striata from treated animals were homogenized in 1% SDS at 95 °C and centrifuged to remove insoluble proteins. By using the bicinchoninic acid technique, the quantity of soluble protein was calculated. Equal quantities of protein (50 μg/lane) were then resolved by SDS-polyacrylamide gel electrophoresis and electroblotted on nitrocellulose.
Results of the study
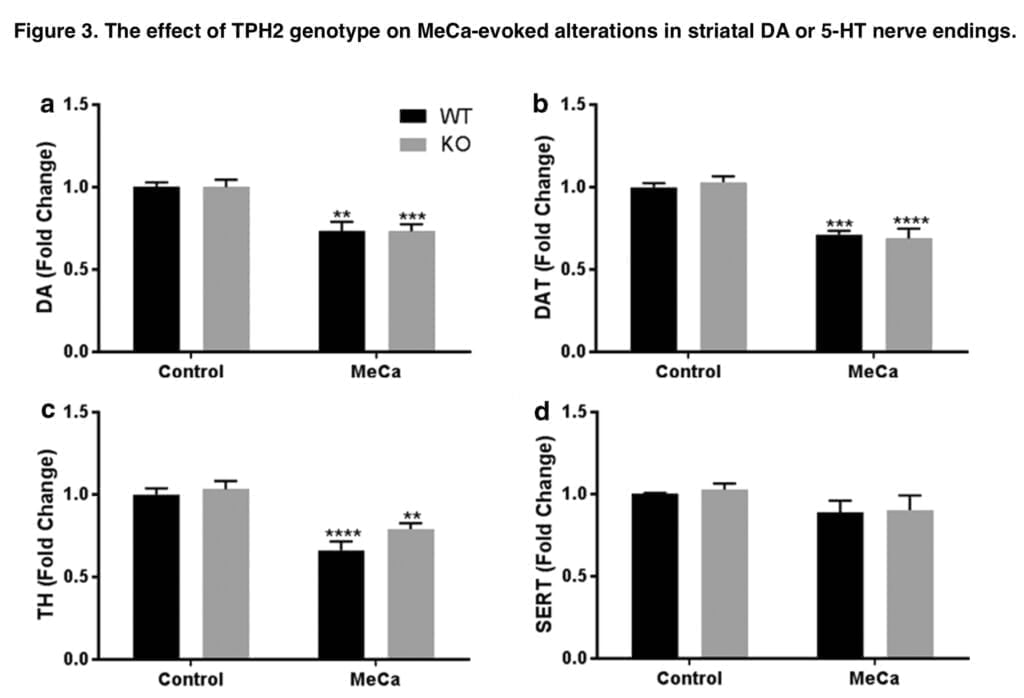
This study’s main goal was to ascertain how 5-HT signaling affected the changes in body temperature brought on by MEPH and its associated molecules, MeCa and 4-MM. We have previously demonstrated that MEPH can result in prolonged hypothermia, with the effects being at their peak shortly after injections of either substance. The well-known hyperthermic effects of METH on body temperature are in stark contrast to our results. The striatal DA nerve terminals are not harmed by mephedrone, and MeCa has far lower levels of neurotoxicity than METH. It seems possible that MEPH- and MeCa-induced hypothermia is “self-protective” against DA nerve ending injury given that preventing METH-induced hyperthermia offers protection against the neurotoxicity of the drug. With this in mind, a secondary goal of our experiment is to determine whether preventing hypothermia can unlock the neurotoxicity of mephedrone and methcathinone.
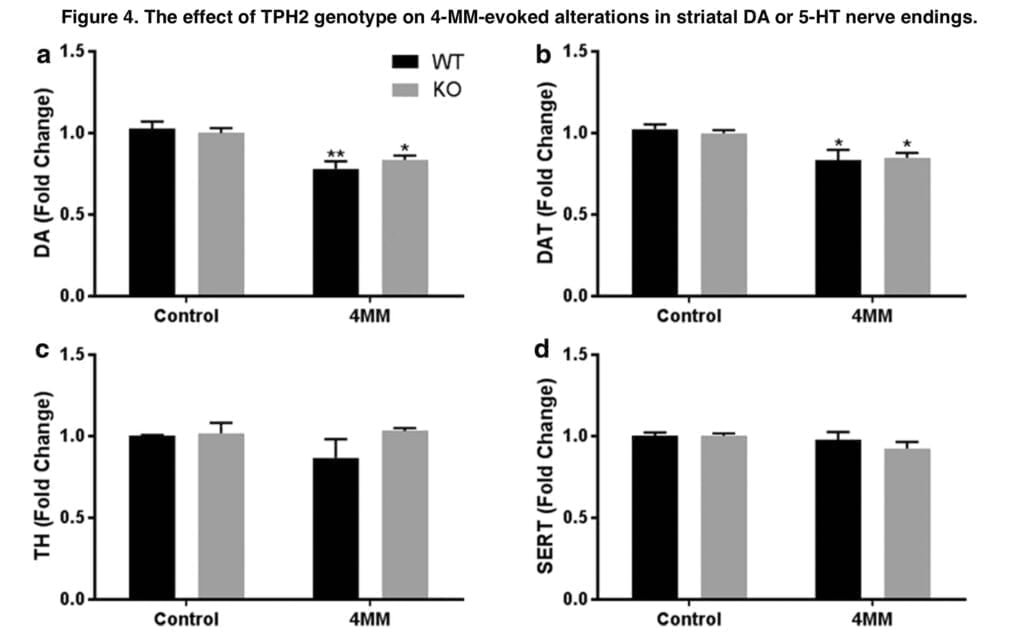
We next looked at the relative neurotoxicity of each substance in both genotypes because the preceding investigation by Shortall and colleagues did not examine whether the removal of hypothermia imparted neurotoxicity to the otherwise nontoxic MEPH. In Tph2-WT mice, MeCa and 4-MM treatment resulted in decreases in DA, DAT, and TH that were comparable to our earlier research and other studies; MEPH, as was predicted, had no such effects. It was assumed that because METH-induced hyperthermia has a neuroprotective impact, eliminating hypothermia in Tph2-KO mice will increase MeCa toxicity as well as impart neurotoxic effects to MEPH. However, there were no appreciable genotype differences in the drug-induced decreases in DA, DAT, or TH, nor was there an appearance of MEPH neurotoxicity, despite the attenuation of MEPH- and MeCa-induced hypothermia in Tph2-KO mice and the rebound hyperthermia. Consequently, it is evident that the hypothermia brought on by MEPH and MeCa has no appreciable effect on the level of neurotoxicity brought on by exposure to these substances. Similarly, 5-HT signaling does not seem to play a significant role in the mechanism of neurotoxicity induced by 4-MM or MeCa. Apart from the indirect hypothermic response, the involvement of 5-HT in neurotoxicity was not anticipated because the majority of studies primarily discount the impact of 5-HT release after drug exposure and instead focus on the oxidative stress caused by excessive DA release by cathinones and amphetamines.
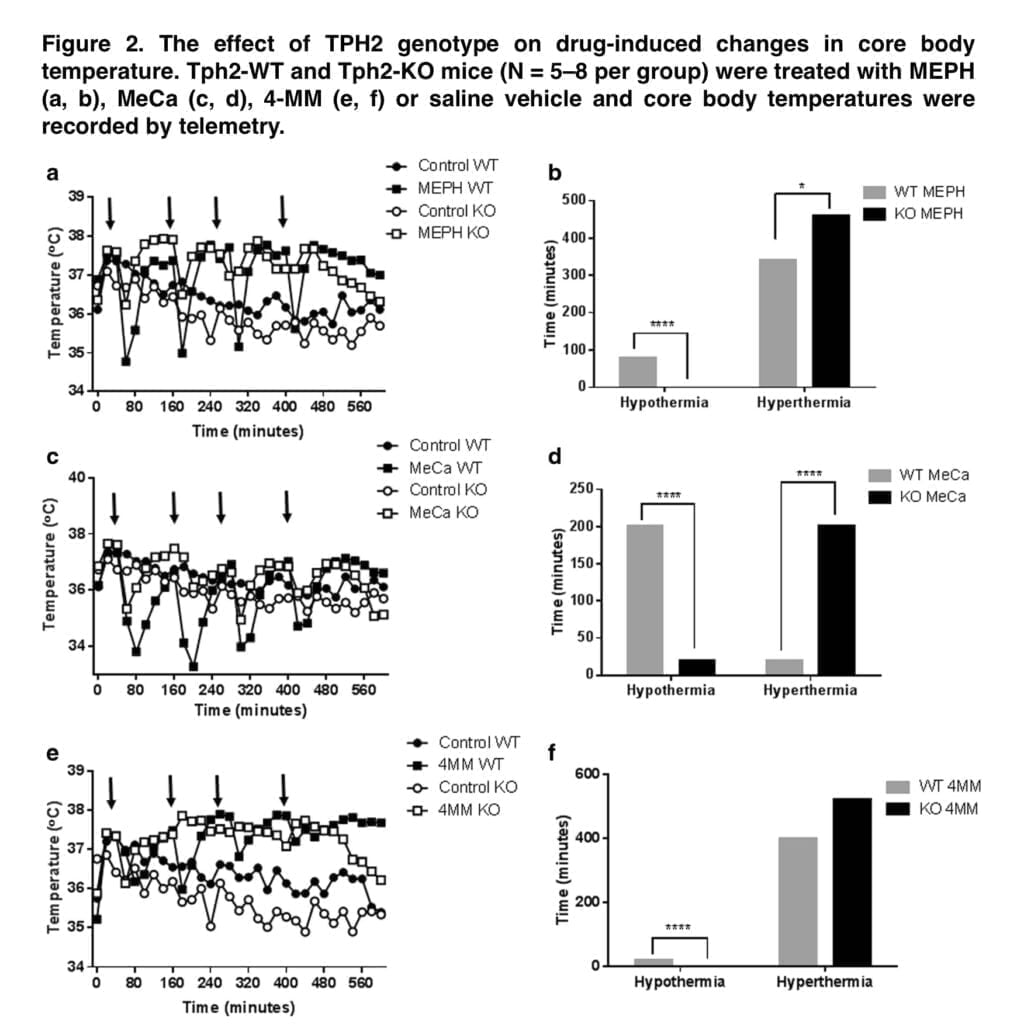
Together, the results of this work demonstrate that 5-HT mediates the hypothermic effects of MEPH and MeCa and that drug-induced hypothermia is unable to explain the lower levels of neurotoxicity generated by MEPH and MeCa compared to METH. The release of endogenous 5-HT is the most reasonable explanation for MEPH- and MeCa-induced hypothermia. Because they lack 5-HT genetically, Tph2-KO mice do not react to MEPH or MeCa by becoming hypothermic. In instance, medications that elicit hypothermia in Tph2 mice result in rebound hyperthermia in Tph2-KO animals. The degree of hypothermia seems to be inversely correlated with this impact. This can be shown in Figure 2, which depicts that following MeCa, there is a higher transition from hypo- to hyperthermia and much less so for MEPH. Therefore, it seems that under normal circumstances, the endogenous 5-HT released by MEPH and MeCa produces hypothermia. In the absence of brain 5-HT, the body temperature response changes into a mild hyperthermia but, at least in the cases of MEPH and MeCa, does not result in the appearance or amplification of neurotoxicity.
Conclusion
Future research must concentrate on the variations between these two substances in additional processes considered to be involved in dopaminergic toxicity. The production of ammonia after liver injury may be a promising process to research. Studies have shown that METH use repeatedly causes liver damage, which is what causes increased ammonia levels. This rise in ammonia causes the brain to produce glutamate, which encourages excitotoxic injury to dopaminergic nerve terminals. Although there is some recent evidence of hepatocellular toxicity, investigations of MEPH and its propensity to raise ammonia levels have not yet been conducted. Future research comparing the effects of MEPH and METH may reveal a major difference in these consequences, which might help us understand the primary route in METH toxicity and, ultimately, develop therapies that can lessen or eliminate the abuse’s long-lasting negative effects.
